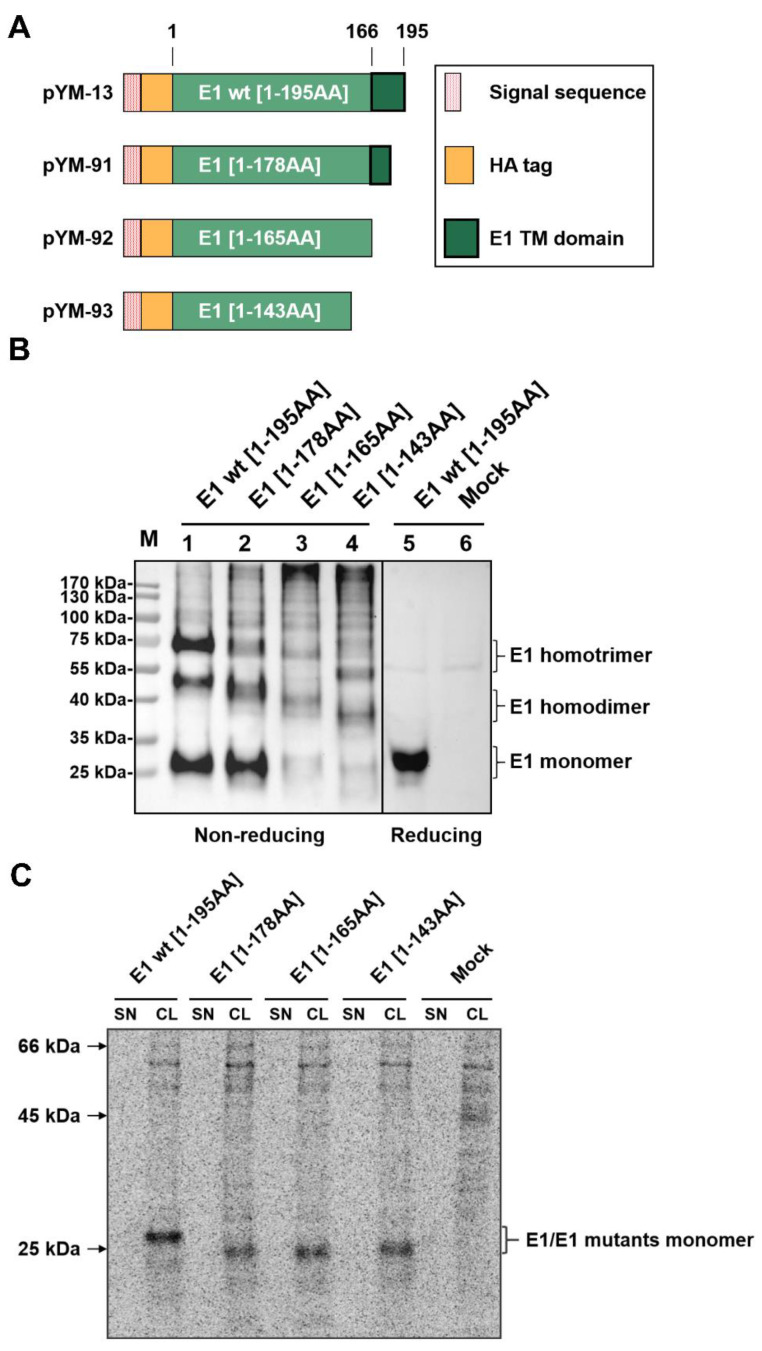
Figure 1.
E1 forms homooligomers independent to its membrane anchor. (A) Schematic representation of HA-tagged E1 wt/C-terminally truncated E1 proteins expressed from the constructs. The box on the right gives the meaning of the differently designed bars [red dotted bar: Erns signal sequence fused to the E1 sequences (green bars) with the length of the sequences given]. A HA-tag (orange bar) was introduced in between the signal sequence and the E1 sequence for the detection of E1. The black frame represents the putative transmembrane region of E1. (B) The HA-tagged E1 and its truncated variants were expressed in RK-13 cells, and expression products were analyzed via western blot on the following day. The cells were lysed with lysis buffer without (left) or with (right) β-mercaptoethanol, aliquots of the lysates loaded onto SDS gels and separated electrophoretically. Western blot analysis was carried out using primary antibody α-HA and PO-labeled second antibodies for the detection of protein. For each lane, the length of the expressed E1 sequence is given on top. Molecular weight of the size marker bands given on the left. As a supplement, we provide a file with the original western blot results to demonstrate that the two parts were derived from one gel. (C) The HA-tagged E1 expression plasmids were expressed in BHK-21 cells using the Vaccinia virus MVA T7 expression system [23,30,31], and the empty pCI empty vector served as a mock control. Expressed proteins were labelled with 35S amino acids. From supernatant (SN) and cell lysates (CL) of the transfected cells, proteins were precipitated with a specific HA-tag antiserum, separated by SDS-PAGE under reducing conditions detected on imaging plates. Molecular weight of the size of the marker bands given on the left.