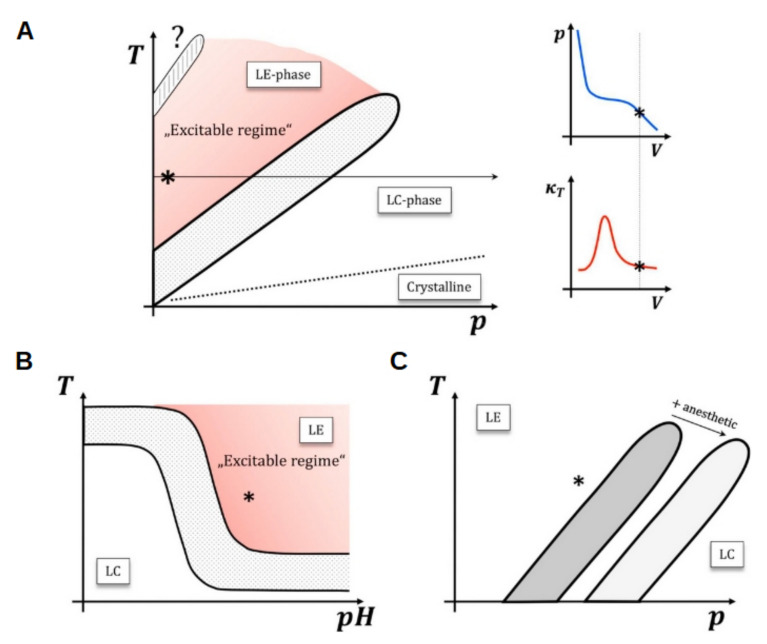
Figure 4.
Scheme showing the relation between membrane phase state and excitability. (A) The cell membrane is excitable if the resting state (asterisk) is in the vicinity of an ordered-disordered transition (e.g., from liquid expanded (LE) to liquid condensed phase (LC) in liquid monolayers; or from lamellar fluid to gel in lipid bilayers). This is illustrated by the P-V isotherm and the derived isothermal compressibility on the right (the arrow illustrates the respective slice through the phase plane). State changes move the system state (asterisk) through phase space and hence change the physical properties of the membrane. At low T / high P the membrane “freezes” into a crystalline-like state. (B) In a T-pH plot, the phase boundary is sigmoidal. The underlying reason for this additional nonlinearity is that the headgroups of membrane molecules are ionizable. This results in a nonlinear change of the transition temperature with pH. Thus, acidification at constant T and P can move the resting state into the LC phase. (C) According to the melting point depression theory [162,166], anesthetics leave the resting state in the disordered phase, but increase its distance to the transition. This figure is reproduced from ref. [164] with permission from Elsevier.