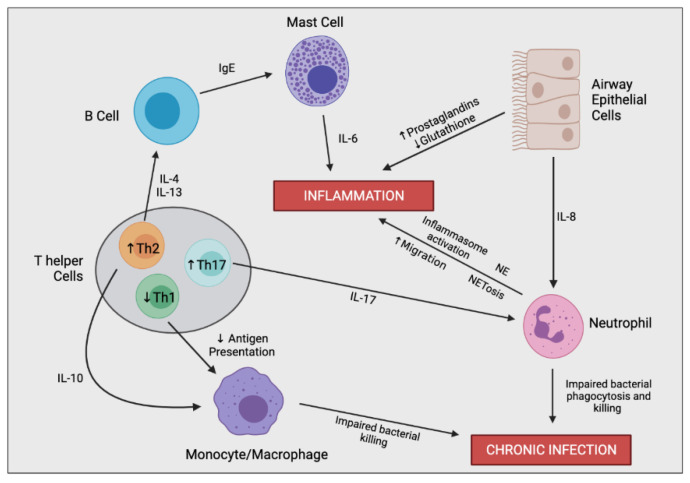
Figure 3.
Simplified schematic outlining the complex immune cell interaction involved in producing the hyperinflammatory response and chronic infection in CF. In the CF airway, airway epithelial cells (AECs) secrete increased levels of IL-8, resulting in enhanced neutrophil migration. Perturbed secretion of prostaglandins and glutathione from AECs promotes inflammation. Neutrophils are the predominant driver of airway inflammation through multiple mechanisms including increased inflammasome activation, increased secretion of neutrophil elastase (NE), and a bias towards neutrophil extracellular trap (NET)-mediated cell death (NETosis). Neutrophils also possess impaired bacterial phagocytosis and killing leading to insufficient bacterial clearance and chronic infection. CF-macrophages also secrete increased levels of IL-8 and other proinflammatory mediators (not shown), promoting neutrophil infiltration and further inflammation directly. CF-macrophages also undergo impaired bacterial killing encouraging persistent infections. T helper cells in CF are skewed towards Th2 and Th17 differentiation rather than a Th1 phenotype. Th17 cells secrete IL-17, promoting neutrophil infiltration, as well as other proinflammatory cytokines which contribute to the hyperinflammatory response (not shown). Th2 cells stimulate a pro-allergic response involving increased secretion of IL-4 and IL-13 leading to IgE production. Th2 cells also secrete IL-10, dampening the expression of co-stimulatory molecules on macrophages causing decreased antigen presentation and impaired bacterial clearance.