Table 2.
Amidation reactions on cCNCs for the design of functionalized CNCs in various applications.
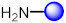
|
Reaction Conditions | Dispersion | DO of Ccnc a | Application | Reference |
|---|---|---|---|---|---|
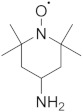
|
EDC, NHS, pH 7.5–8 24 h, 50 °C |
CHCl3, THF, toluene | 0.21 | Nanocomposites | [69] |
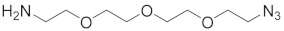
|
EDC, NHS, MES pH 4, 24 h, RT |
Ionic liquid | 0.2–0.28 | Click chemistry Nanoplatelet gels |
[70] |
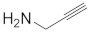
|
EDC, NHS, MES pH 4, 24 h, RT |
Ionic liquid | 0.2–0.28 | Click chemistry Nanoplatelet gels |
[70,71] |
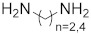
|
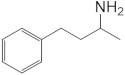
EDC, NHS, MES pH 5, 24 h, RT |
Aqueous | 0.25 | Conjugation | [72] |
|
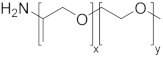
EDC, NHS, pH 8–8.5 72 h, RT |
Acetone CH2Cl2 |
0.22 | Nanocomposites | [74] |
|
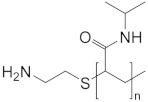
EDC, NHS, pH 7.5–8 24 h, RT |
DMF/aqueous | 0.2 | Thermo-responsive materials | [75] |
|
EDC, NHS, pH 7.5–8 48 h, RT |
Aqueous | 0.25 | Thermo-responsive materials | [76] |
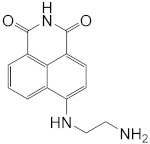
|
EDC, NHS, pH 7.2, PBS, DMF16 h, RT | DMF/aqueous | 1.52 b | Nanosensors | [77] |
| Quantum Dots-NH2 | EDC, NHS, pH 5, overnight, RT | Aqueous | 0.24 | Bioimaging | [78] |
| Amino-functionalized sugars | EDC, NHS, MES pH 7.5–8.5 16 h, RT |
Aqueous | 1.56 b | Biorecognition | [79] |
| Fluorescent quinoline dye | EDC, NHS, MES pH 7.5–8.5 16 h, RT |
Aqueous | 1.56 b | Bioimaging | [79] |
| Aminoethyl rhodamine dye | CDI, DMF, 20 h, 60 °C |
DMF/aqueous | 1.102 b | Molecular switching, imaging | [80] |
| Lysozyme-NH2 | EDC, NHS, MES pH 5.7, 3 h, RT |
Aqueous | - | Antibacterial | [81] |
| Lysozyme-NH2 | DMTMM, 1 h, 37 °C | Aqueous | 0.7–0.9 b | Emulsifier | [66] |
a The values displayed for the DO or carboxyl content of cCNC correspond to values prior to the amidation reactions; b Carboxyl content (mmol/g); EDC: N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride; NHS: N-hydroxysuccimide; MES: 2-(N-morpholino)-ethanesulfonic acid buffer; DMTMM: 4-(4,6-dimethoxy-1,3,5-tr,azin-2-yl)-4-methylmorpholinium chloride; CDI: 1,1′-carbonyldiimidazole.
