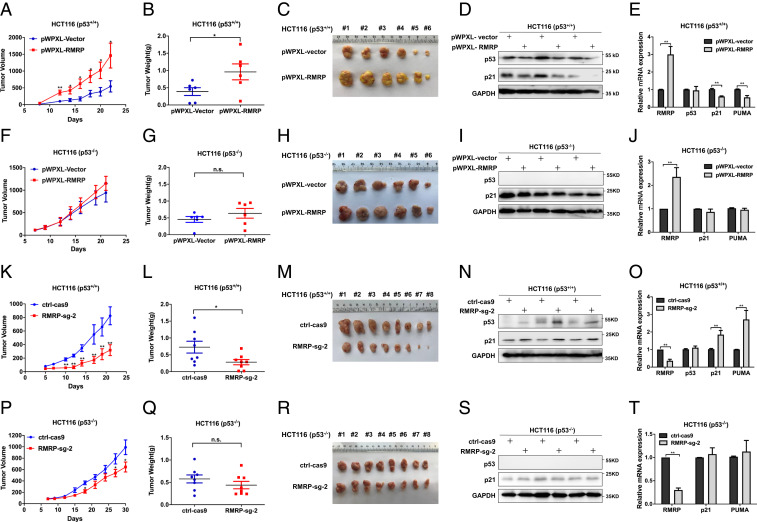
Fig. 4.
RMRP endorses tumor growth in vivo by inactivating p53. (A) Lentivirus-based overexpression of RMRP in HCT116 p53+/+ cells significantly elevates tumor volume in average compared with the control group. (B and C) The dissected tumors show that RMRP overexpression increases the weight and mass of tumors derived from HCT116 p53+/+ cells. Data are represented as mean ± SD, n = 6. (D) Overexpression of RMRP inhibits p53 and p21 protein expression in vivo. (E) Overexpression of RMRP inhibits the mRNA expression of p21 and PUMA examined in three pairs of xenograft tumors (mean ± SD). (F–H) Overexpression of RMRP has a marginal effect on the growth of tumors derived from HCT116 p53−/− cells. Data are represented as mean ± SD, n = 6. (I and J) Overexpression of RMRP does not affect p53 target gene expression in three pairs of xenograft tumors derived from HCT116 p53−/− cells (mean ± SD). (K) CRISPR/ Cas9–mediated depletion of RMRP in HCT116 p53+/+ cells significantly suppresses tumor volume in average compared with the control group. (L and M) The dissected tumors show that knockout of RMRP diminishes the weight and mass of tumors derived from HCT116 p53+/+ cell. Data are represented as mean ± SD, n = 8. (N) RMRP knockout bolsters p53 and p21 protein expression in vivo. (O) RMRP knockout activates the mRNA expression of p21 and PUMA examined in three pairs of xenograft tumors (mean ± SD). (P–R) Knockout of RMRP has a marginal effect on the growth of tumors derived from HCT116 p53−/− cells. Data are represented as mean ± SD, n = 8. (S and T) Knockout of RMRP does not affect p53 target gene expression in three pairs of xenograft tumors derived from HCT116 p53−/− cells (mean ± SD). *P < 0.05, **P < 0.01 by two-tailed Student’s t test. n.s. indicates no significance.