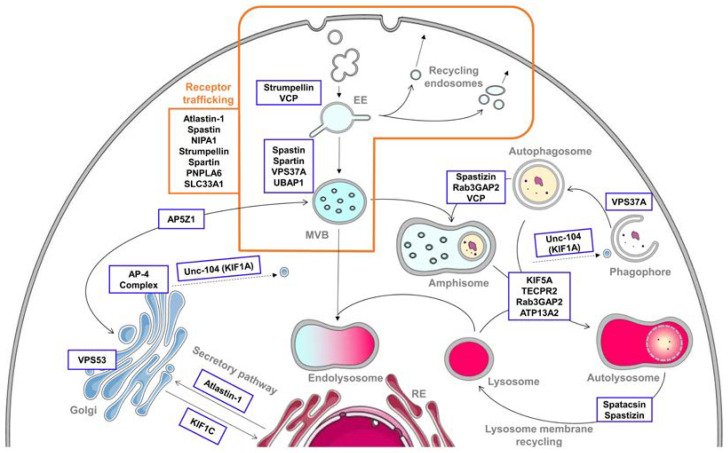
Figure 1.
Implication of hereditary spastic paraplegia (HSP) proteins in endolysosomal and autophagic pathways. The endolysosomal pathway begins with endocytosis, consisting in the uptake of extracellular contents into vesicles. They fuse with early endosomes (EEs), which mature into multivesicular bodies (MVBs). During this fusion and maturation, the cargoes are sorted and can have three different fates. They can be directly recycled back to the plasma membrane via recycling endosomes, sent to the trans-Golgi network (TGN) or delivered to lysosomes for degradation. This process is notably highly involved in receptor trafficking, where numerous HSP proteins are implicated. For the degradation, the MVB has to fuse with the lysosome to form an endolysosome. However, MVBs can also fuse with autophagic compartments for their maturation, forming an amphisome. This hybrid structure will merge with a lysosome to give an autolysosome, where the degradation will take place, highlighting the importance of interconnections between endocytosis and autophagy. Autophagy involves the sequestration of cytoplasmic contents into a double-membrane vesicle called autophagosome and their degradation by fusion of the autophagosome with lysosomes to form an autolysosome. Once the degradation is effective, new lysosomes have to be formed using the lysosomal membrane components present in the autolysosome by the lysosome membrane recycling. All these compartments are highly interconnected, especially the Golgi and endoplasmic reticulum (ER), which also communicate with each other along the secretory pathway. Numerous HSP proteins, framed on the figure, are involved in various steps of the endocytic and autophagic pathways. Their precise involvement is summarized in Table 1. Of note, some elements of the figure are adapted from Servier Medical Art by Servier licensed under a Creative Commons Attribution 3.0 Unported License.