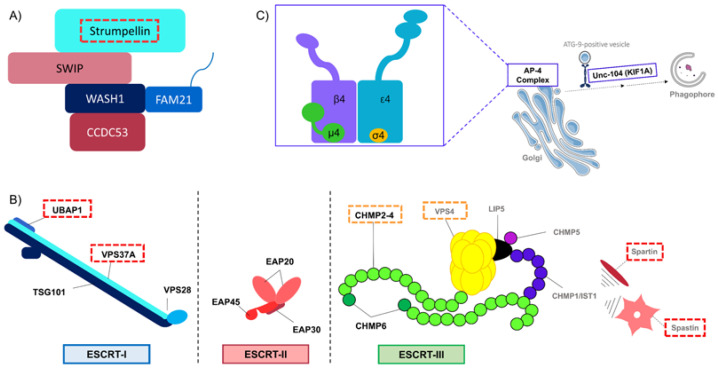
Figure 2.
Schematic representations of the WASH, ESCRT-I to –III and AP-4 complexes. (A) Schematic representation of the WASH complex. The WASH complex is composed of five subunits: strumpellin (or WASHC5), SWIP, WASH1, CCDC53 and FAM21. The WASH complex plays a critical role in endosome tubulation and fission. Mutations in the WASHC5/strumpellin subunit (highlighted by a dashed red frame) led to a pure form of HSP, SPG8. (B) Schematic representation of the ESCRT system from ESCRT-I to ESCRT-III. The ESCRT system plays a central role in intracellular trafficking. Notably, the ESCRT machinery is involved in the formation of MVBs since they are implicated in the sorting of cargoes and the formation of ILVs. ESCRT-I is a tetrameric complex composed of four subunits with two core subunits: TSG101 and VPS28 and of two additional subunits: MVB12A/B, UBAP1, UBA1L or UMAD1 and one isoform of VPS37 (A/B/C/D). ESCRT-II is also a tetramer composed of EAP45, EAP30 and two EAP20. Finally, ESCRT-III is composed of four main proteins, CHMP2A/B, CHMP3, CHMP4A/B/C, and CHMP6. To those proteins, can be associated at least three ESCRT-III related proteins: CHMP1A/B, CHMP5 and IST1. On the right panel are also represented VPS4 and its cofactor LIP5 as well as spastin and spartin which are interactors of ESCRT-III and ESCRT-III related proteins. Importantly, VPS37A, UBAP1, spastin and spartin are mutated in HSP, corresponding to SPG53, SPG80, SPG4 and SPG20 respectively. Of note, mutations in the gene encoding for CHMP2B can give rise to other neurodegenerative conditions: amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD) and ALS-FTD. In addition, mutations in one isoform of VPS4, VPS4A, has been found in a multisystemic disease with abnormal neurodevelopment. The proteins mutated in HSP or other neurological disorders are highlighted by dashed red or orange frames, respectively. (C) Schematic representation of the AP-4 complex and ATG-9-positive vesicles transport. The AP-4 complex specifically localizes to the TGN where it would allow the sorting of transmembrane proteins into cargoes and the recruitment of their transport machineries for their delivery to their destined localization. Among those proteins can be found ATG9A in mammals that will be delivered to the phagophore in ATG9A-positive vesicles. In the zoom box, there is a representative schematic model of AP-4 with the four subunits composing the complex: ε4, β4, μ4 and σ4, respectively encoded by AP4E1, AP4B1, AP4M1 and AP4S1. Mutations in these four genes lead to severe neurodevelopmental forms of HSP (SPG47, SPG50, SPG51 and SPG52, respectively). In C. elegans, the motor protein Unc-104, the homologue of KIF1A, mutated in SPG30, would participate in the transport of ATG-9-positive vesicles to the phagophore for the autophagosome biogenesis. Of note, some elements of the figures are adapted from Servier Medical Art by Servier licensed under a Creative Commons Attribution 3.0 Unported License.