Table 2.
Blood–brain barrier in vitro models (organ-on-chip, hydrogel model, and vascularized brain organoids).
| Citation | Experiment Conditions * | Main Readouts ** |
|---|---|---|
| Organ on chip | ||
| Wang et al. 2017 [236] |
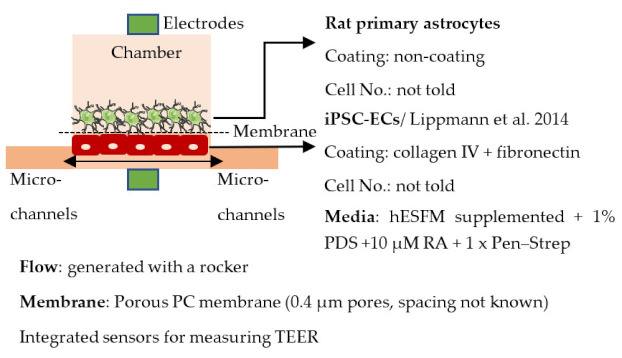
|
Max TEER (Ω× cm2) Monoculture: 368 Co-culture: 4399 Permeability, Dextrans (Papp× 10−8 cm/s) Co-culture: 8.43 (4 kDa), 2.18 (20 kDa) and 0.982 (70 kDa) |
| Vatine et al. 2019 [237] |

|
Max TEER (Ω × cm2) Co-culture (+neural): 1500 Permeability, Dextran (Papp × 10−7 cm/s) Monoculture: ~3 (exact values not told) Co-cultures (+astrocyte, pericytes/ + neural): ~1 (exact values not told) Other ↓ ZO1 expression and ↓ permeability to Dextran after cytokine exposure (+astrocytes, pericytes) Spontaneous neuronal activity (+neural) |
| Park et al. 2019 [238] |
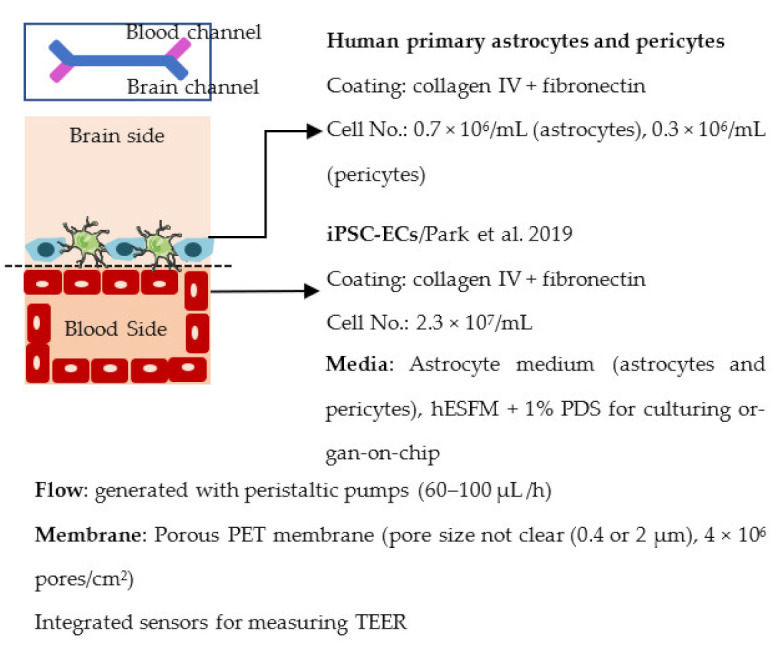
|
Impedance (Ω) ~25 000 Permeability, Dextran (Papp × 10−8 cm/s) 8.9 (3 kDa), 1.1 (10 kDa) and 0.24 (70 kDa) Other Expression and functionality of efflux transporters |
| Motallebnejad et al. 2019 [239] |

|
Max TEER (Ω × cm2) 1590 (0.4 µm membrane), 1369 (8.0 µm membrane) Co-culture increased TEER values Permeability, NaF (× 10−6) Below 1 Other Efflux transporter activity, decreased TEER after TGF-β1 exposure |
| Pediaditakis et al. 2020 preprint [240] |
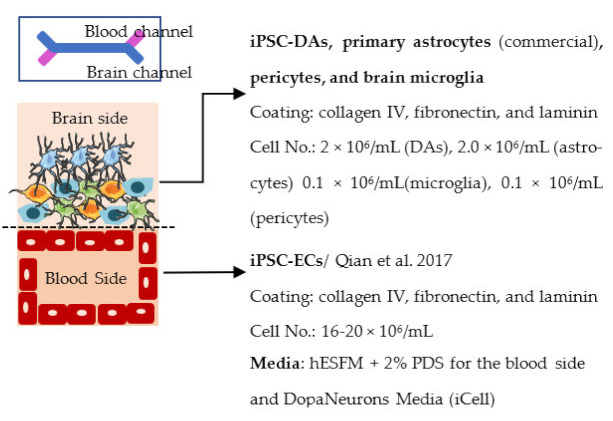
|
Permeability (Papp × 10−6 cm/s) range 1–3 (3 kDa), 4–6 (LY) Other RNAseq (brain side): more mature phenotype in the chip compared to conventional cell culture, chip recapitulated gene expression profile of primary tissue |
| Hydrogels/3D vessels | ||
| Campisi et al. 2018 [243] |
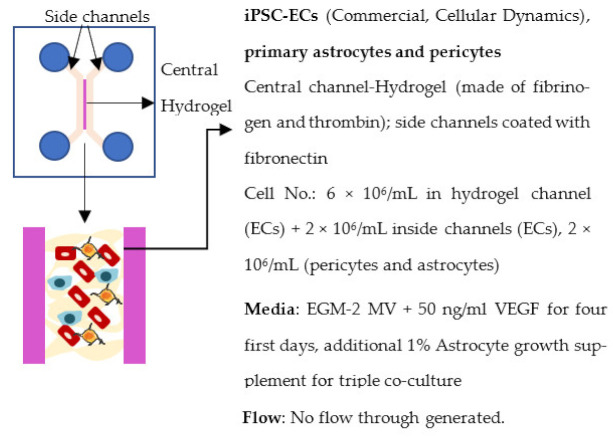
|
Permeability coefficient, Dextran (× 10−7 cm/s) Monoculture: 6.6 (40 kDa), 12 (10 kDa) Co-culture (+pericytes): 2.5 (40 kDa), 4.8 (10 kDa) Triple co-culture (+pericytes, astrocytes): 0.89 (40 kDa), 2.2 (10 kDa) Other Complex and branched vascular network, ↑ ZO-1, claudin-5 and occludin expression in triple co-culture, ↑ gene expression of several transporters in triple co-culture |
| Faley et al. 2019 [244] |
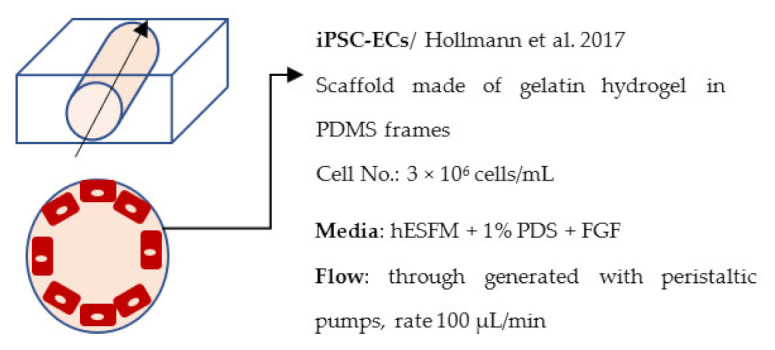
|
Permeability, Dextran 3 kDa (× 10−7 cm/s) Day 1: 1,2 (static), 1,9 (perfused) Day 7: 4.6 (static), 1.4 (perfused) Day 14: 11.7 (static), 0.23 (perfused) |
| Blanchard et al. 2020 [107] |
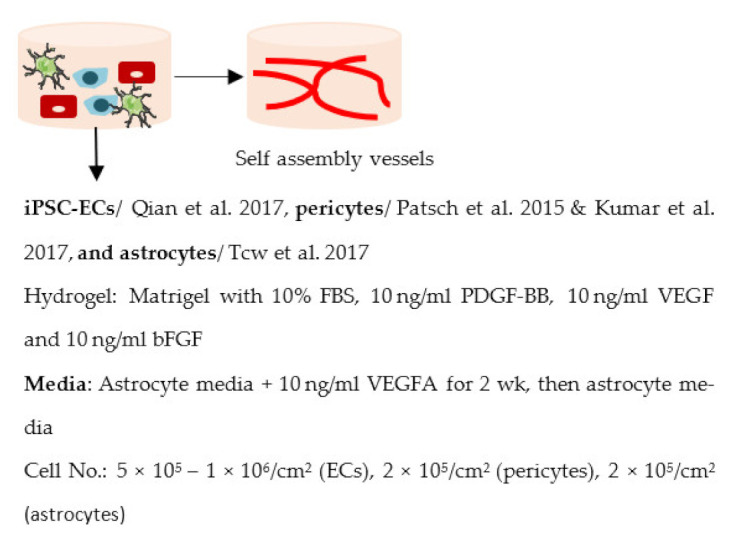
|
Other Capillary structure ↑ CLDN5, JAMA, PGP, LRP1, RAGE and GLUT1 expression in co-culture |
| Vascularized organoids | ||
| Pham et al. 2018 [245] |
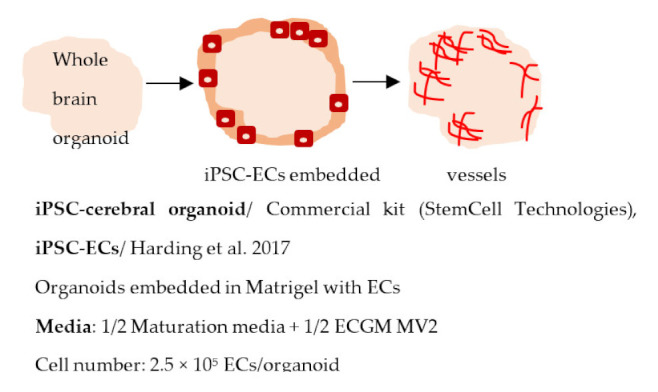
|
Other Tubular structures in organoids, positive for CD31 |
| Cakir et al. 2019 [246] |
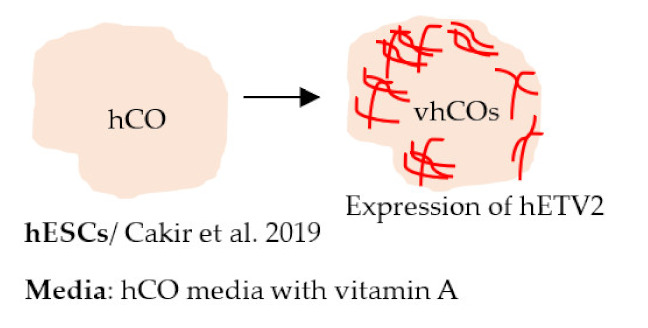
|
TEER (Ω × cm2) Day 30: 186 (vhCO), 135 (hCO) Day 70: 351 (vhCO), 71 (hCO) Other Presence of vascular tubes positive for CD31, expression of BBB markers (ZO-1, occludin) Presence of astrocytes and pericytes |

| ||
Abbreviations: ECs—Endothelial cells; ESC—Embryonic stem cell; FBS—Fetal bovine serum; FGF—Fibroblast growth factor; hCO—Human cortical organoids; hESFM—Human endothelial serum free media; hETV2—Human ETS variant 2; LY—Lucifer yellow; NaF—Sodium fluoride; NPC—Neural progenitor cell; PDGF—Platelet derived growth factor; PDMS—Polydimethylsiloxane; PDS—Platelet-poor plasma-derived serum; PET—Polyethyl terephthalate; TEER—Transendothelial electrical resistance; VEGF—Vascular endothelial growth factor; VEGFA—Vascular endothelial growth factor A; vhCO—Vascularized hCO; Pen-Strep—Penicillin–streptomycin; PC—Polycarbonate; * Describing co-culture conditions, unless otherwise mentioned; ** Only results from healthy lines included.
