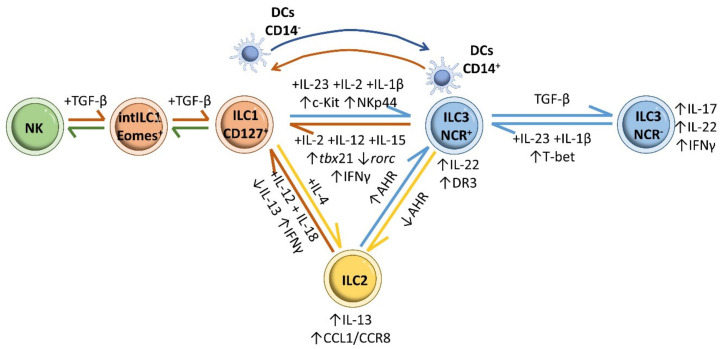
Figure 8.
ILC plasticity. Prolonged exposure of ILC3s to the type 1 polarizing cytokines IL-2, IL-12 and IL-15 induces their conversion to CD127+ ILC1 cells, with downregulation of RORC, upregulation of TBX21 and IFN-γ production. Reverse differentiation of ILC1s to ILC3s is driven by IL-23, IL-2 and IL-1β. Moreover, CD14− DCs mediate the transformation of ILC1s into ILC3s in vivo by promoting synthesis of c-kit and NKp44 in ILC1s, whereas CD14+ DCs are implicated in the transformation of NCR+ ILC3s into ILC1s. IL-23 and IL-1β stimulation induces the conversion of NCR− ILC3s into the more proinflammatory NCR+ ILC3 subset through T-bet upregulation. The reverse differentiation from NCR+ ILC3s to NCR− ILC3s is promoted by high levels of TGF-β. Removal of AHR enriches the effect of intestinal ILC2s, whereas increased AHR expression restrains ILC2 function while increasing ILC3 function. Treatment with IL-12 and IL-18 reduces IL-13 expression in ILC2s, shifting them to an IL-13− IFN-γ+ ILC1 phenotype. ILC2-derived ILC1s can revert to ILC2s in response to IL-4. NK cells (CD49a− CD49b+ Eomes+) differentiate into intermediate type 1 innate lymphoid (intILC1, CD49a+ CD49b+ Eomes+) populations and ILC1s (CD49a+ CD49b− Eomesint) in response to cytokine and TGF-β signaling.