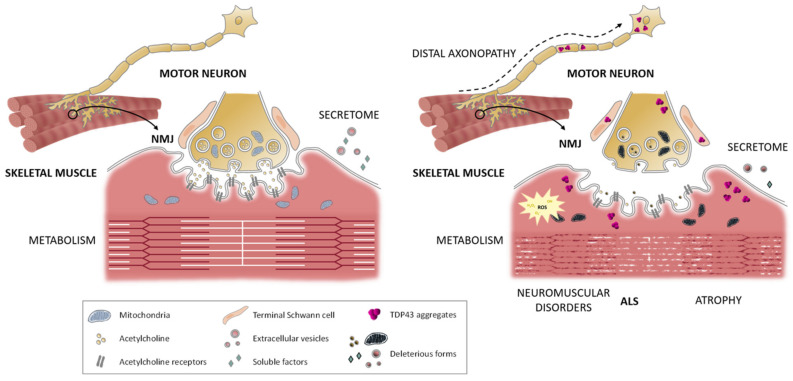
Figure 1.
Proposed model for the skeletal muscle as a key contributor on ALS peripheral etiopathogenesis through a “dying back” mechanism. The main components of the neuromuscular junction (NMJ) are depicted for healthy (left) and ALS (right) states, respectively: the pre-synaptic part (nerve terminal), post-synaptic part (motor endplate), and the synaptic cleft in between the two. As illustrated, NMJs in ALS are destabilized, and different components are altered. Indeed, early metabolic and myogenic defects in skeletal muscle trigger mitochondrial dysfunction, oxidative stress, and increased production of reactive oxygen species (ROS). Besides, dysregulated protein homeostasis enhances the presence of cytoplasmic and nuclear aggregates, including TDP-43 inclusions in muscle, that are also present in other cell types, including terminal Schwann cells and motor neurons (MN). Moreover, the skeletal muscle increases extracellular vesicles production (secretome), which may content toxicity and release it to other NMJ cell components. All these muscle alterations result in muscle atrophy and NMJ dismantlement, which are classical hallmarks of ALS. Thereby, altered intercellular communication exacerbates nerve retraction and degeneration of the distal MN ending, which is spread in a retrograde manner (dashed black arrow) into the soma, resulting in progressive MN degeneration.