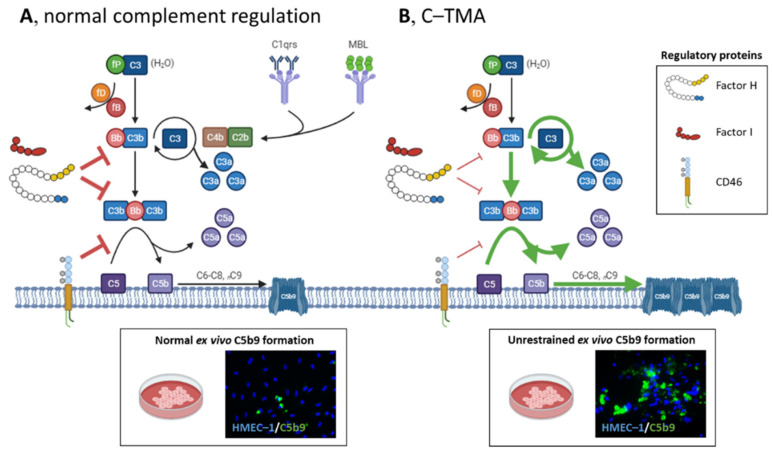
Figure 2.
Schematic overview of complement activation and regulation in health and disease. (A) The complement system can be initiated via the classical (C1qrs), lectin (MBL), and alternative pathways (C3), converging to C3. The alternative pathway is a spontaneously and continuously active surveillance system operating in the circulation and on the cell surface. C3 (H2O) binds factor B (fB) and factor D (fD), and the latter cleaves fB into Bb, the serine esterase that cleaves C3 into C3a and C3b. C3’s thioester domain located in C3b can bind to the cell surface (e.g., microbes), providing a platform to form the C3 convertase of the alternative pathway (i.e., C3Bb) to cleave more C3, activating an amplification loop. Next, additional C3b can shift the C3 convertase to a C5 convertase, cleaving C5 into C5a and C5b, activating the terminal complement pathway. C5a and, to a lesser extent, C3a attract leukocytes to the site of complement activation. C5b can bind C6, C7, C8, and various C9 molecules to form the lytic C5b9 (i.e., membrane attack complex) on cells. Host cells, including the endothelium, are protected from the harmful effects of complement activation by factor I, factor H, and CD46 (also known as membrane cofactor protein); these proteins have decay-accelerating and cofactor activities, leading to factor I-mediated cleavage of C3b into inactivated proteins. (Normal ex vivo C5b9 formation on perturbed human microvascular endothelial cells of dermal origin (HMEC–1) indicates normal complement regulation.). (B) In C-TMA, rare variants in complement genes (i.e., loss of function of factor I, factor H, or CD46 (thin red lines); gain of function of C3 or CFB (green lines)) and/or autoantibodies targeting complement regulatory proteins result in unrestrained complement activation, formation of C5b9 on the endothelium, and a procoagulant environment that triggers thrombosis. (Massive ex vivo C5b9 formation on perturbed HMEC–1 indicates unrestrained C5 activation.) fP, properdin.