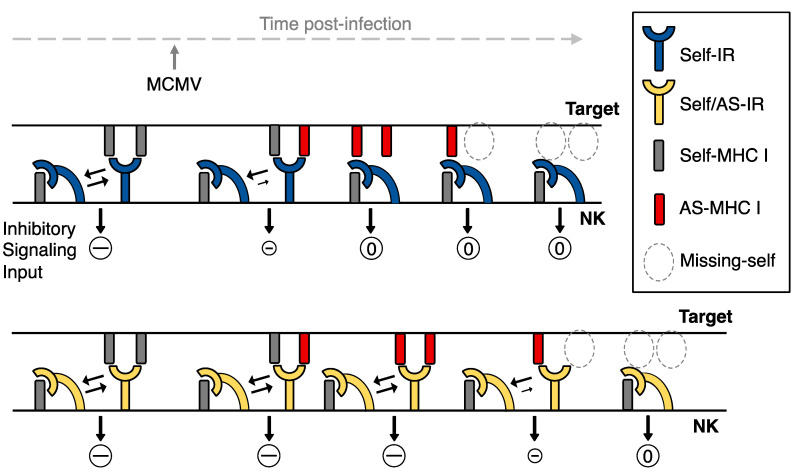
Figure 1.
A model for IR-licensed NK sensing of virus infected cells. At steady-state, an equilibrium defines the number of Ly49 IRs engaged in cis or in trans with self-MHC I molecules and IR negative signaling (–) in NK cells contributes to self-tolerance. Over the course of viral infection, altered-self (AS) MHC I molecules may accumulate at the target cell surface, along with downregulation of surface MHC I (i.e., missing-self). For IRs that are highly specific for self-MHC I relative to altered-self, cis-binding may prevail during interaction with infected targets, thereby diminishing negative signaling (0) so that NK cells bearing activation receptors for ligands on infected targets are triggered. However, IRs that can bind self and AS MHC I may continue to dominantly block activation receptor signaling pathways. Only a sufficient loss of self-MHC I then can trigger both types of IRs. Hence, distinct IRs for self-MHC I, like their activation receptor counterparts, may differ in NK sensing of virus infected targets.