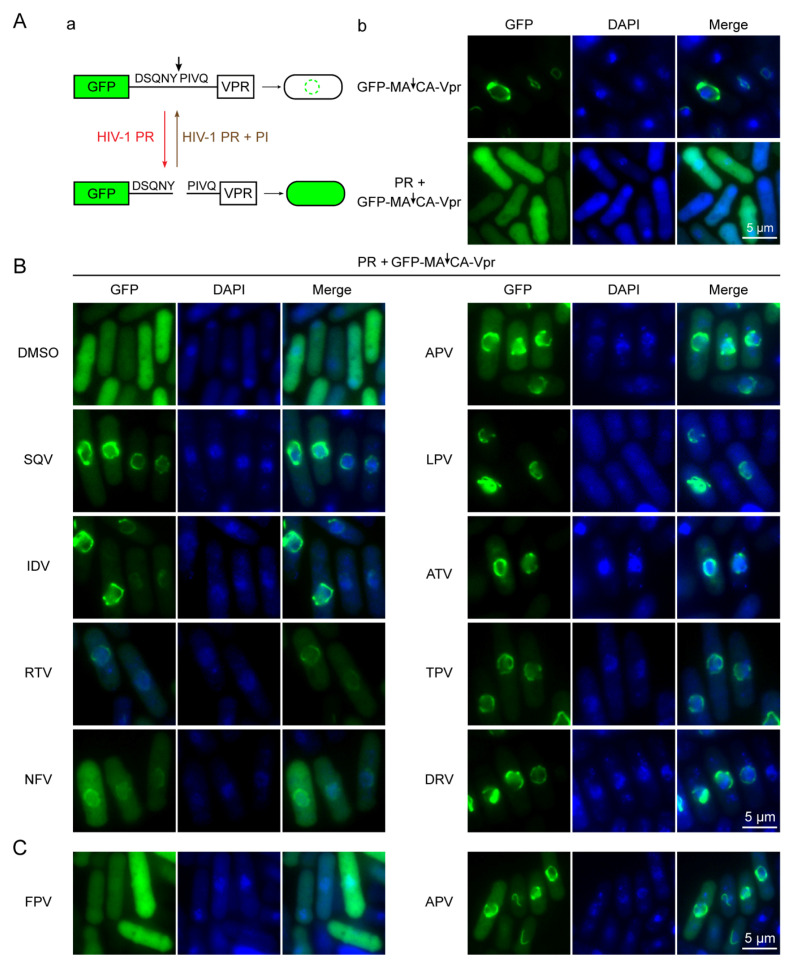
Figure 4.
FDA-approved PI drugs prevent HIV-1 PR enzymatic cleavages of its nature HIV-1 substrate in fission yeast. (A) The schematic shows how the HIV-1 PR enzymatic assay is measured (a) with corresponding and expected results (b). (B) Results of each drug treatment. A total of 150 μM of each drug was used. Images were taken at 20 h post-gene induction. (C) Showing different results between the prodrug FPV (left) and drug APV (right). The measurement of HIV-1 PR-mediated proteolytic cleavage of a natural HIV-1 matrix-capsid (MA↓CA) substrate (DSQNY↓PIVQ) in fission yeast has been described previously [25,32]. Arrow indicates the PR cleavage site. GFP normally distribute uniformly throughout cells [39,40]. HIV-1 viral protein R (Vpr) localizes predominantly on the nuclear membrane and appears as a “ring-like” structure [39,40]. HIV-1 PR-mediated proteolytic cleavage of GFP-MA↓CA-Vpr will show uniform distribution of GFP; prevention of a PI drug on HIV-1 PR-mediated proteolytic cleavage will retain as a green “ring-like” structure, the same as without the production of HIV-1 PR [39,40].