Table 2.
Plk1 inhibitors in clinical trials 1.
| Compound | Clinical Trials |
Current Status |
Conditions | Interventions | Outcomes 2 | Refs. |
|---|---|---|---|---|---|---|
BI 2536
|
11 Clinical trials | Phase I/II 10 Completed 1 Terminated |
Advanced Solid Tumors, Acute Myeloid Leukemia, Non-Hodgkin’s Lymphoma | Monotherapy | Best responses were CR for acute myeloid leukemia and non-Hodgkin’s lymphoma; PR for non-small-cell lung cancer, pancreatic cancer, acute myeloid leukemia, and non-Hodgkin’s lymphoma | [40,41,42,43,44] |
| Combination with Pemetrexed and Gemcitabine | Best response was PR for adenocarcinoma and squamous cell carcinoma | |||||
BI 6727 (Volasertib)
|
26 Clinical trials | 25 Phase I/II 12 Completed 4 Terminated 6 Withdrawn 3 Ongoing |
Acute Myeloid Leukemia Pediatric Patients with Advanced Cancers, Myelodysplastic Syndromes, Non-Hodgkin’s Lymphoma, Urothelial, Ovarian, Lung Pancreatic, Colorectal and Prostate Cancer | Monotherapy | Best responses were CR for acute myeloid leukemia; PR for non-small cell lung cancer, melanoma, ovarian cancer, acute myeloid leukemia, gastric cancer, and urothelial cancer | [46,47,48,49,50,51,52,53,54,55] |
| Combination with Cytarabine, Pemetrexed, Azacitidine, Afatinib, Decitabine, Daunorubicin, Nintedanib, Mitoxantrone and Itraconazole | Best responses were CR for breast cancer in combination with Nintedanib, PR for non-small-lung cancer in combination with Pemetrexed, Nintedanib, Afatinib, and Carboplatin, PR for head and neck carcinoma in combination with Afatinib, PR for undifferentiated follicular dendritic reticulum cell sarcoma and differentiated follicular dendritic reticulum cell retroperitoneal sarcoma in combination with Cisplatin, PR for differentiated hypopharynx carcinoma in combination with Carboplatin | |||||
| 1 Phase III Ongoing | Acute Myeloid Leukemia | Combination with Cytarabine | - | |||
ON 01910.Na (Rigosertib)
|
36 Clinical trials | 32 Phase I/II 24 Completed 1 Terminated 2 Withdrawn 3 Recruiting/ Planned 2 Ongoing |
Refractory Leukemia, Myelodysplastic Syndrome, Squamous Cell Carcinoma Acute/Chronic Myeloid Leukemia, Ovarian and Lung Cancer, Advanced Solid Tumors | Monotherapy | Best response was PR for non-Hodgkin’s lymphoma, thymic cancer, and pancreatic ductal adenocarcinoma | [57,58,59,60,61,72,73] |
| Combination with Nivolumab, Cisplatin, Azacitidine, Oxaplatin, Gemcitabine and Irinotecan | Best responses were CR and mCR for myelodysplastic syndrome in combination with Azacitidine | |||||
| 4 Phase III 3 Completed 1 Ongoing |
Metastatic Pancreatic Adenocarcinoma, Myelodysplastic Syndromes | Monotherapy | Best responses were SD and mCR for myelodysplastic syndromes | |||
| Combination with Gemcitabine | Best response was PR for pancreatic adenocarcinoma | |||||
GSK461364
|
NCT00536835 | Phase I Completed |
Non-Hodgkin Lymphoma, Advanced Solid Malignancies |
Monotherapy | Best response was SD for esophageal and ovarian cancers and endometrial carcinoma | [63] |
MK-1496
|
NCT00880568 | Phase I Completed |
Advanced Solid Tumors | Monotherapy | Best response was PR for parotid gland carcinoma and small cell lung cancer | [64] |
TAK-960
|
NCT01179399 | Phase I Terminated |
Advanced Nonhematologic Malignancies | Monotherapy | Discontinued strategically by sponsor due to lack of efficacy | - |
NMS-1286937/Onvansertib
|
4 Clinical trials | Phase I/II 1 Completed 3 Recruiting |
Advanced or Metastatic Solid Tumors | Monotherapy | Best response was SD for colorectal cancer, pancreatic carcinoma with a K-RAS mutation, head and neck squamous cell carcinoma, and basal cell carcinoma | [68] |
| Acute Myeloid Leukemia, Metastatic Prostate Cancer, Metastatic Colorectal Cancer with a KRAS mutation | Combination with Decitabine, Cytarabine, Abiraterone, Prednisone, FOLFIRI and Bevacizumab | - | ||||
| TKM-080301 (siRNA) |
3 Clinical trials | Phase I/II Completed |
Hepatocellular Carcinoma, Colorectal, Pancreas, Breast and Ovarian Cancer with Hepatic Metastases, Adrenocortical Carcinoma, Neuroendocrine Tumors |
Monotherapy | Best response was PR for adrenocortical carcinoma | [69,70] |
CYC 140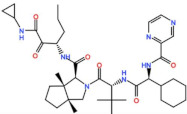
|
NCT03884829 | Phase I Recruiting |
Acute Myeloid Leukemia, Myelodysplastic Syndromes, Acute Lymphoblastic Leukemia |
Monotherapy | - | - |
1 Data collected from clinicaltrials.gov. 2 CR, complete remission; mCR, marrow complete remission; PR, partial response; SD, stable disease.
