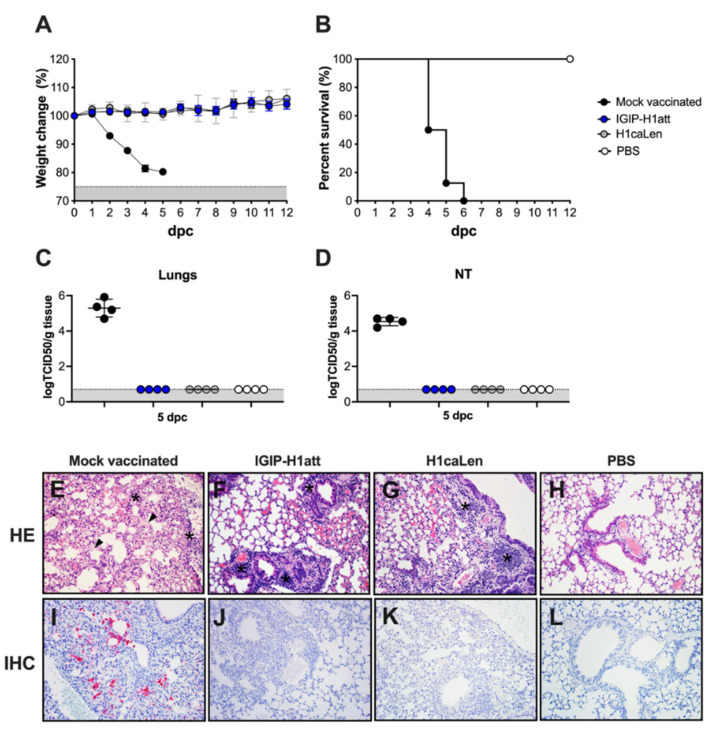
Figure 3.
Efficacy of IGIP-H1att against H1N1 lethal challenge in DBA/2 J mice. Mice (n = 12/group) previously mock-vaccinated were mock challenged (white circles) or challenged (black circles) with 1 × 106 TCID50/mouse of Ca/04 (H1N1). Mice previously vaccinated with IGIP-H1att (blue circles) or H1caLen (grey circles) were challenged similarly. (A) Weight changes (Grey area represents mice reaching humane endpoints) and (B) Survival were monitored for 12 days. At 5 dpc, mice (n = 4/group) were humanely euthanized, and the viral load was evaluated in tissue samples from (C) lungs and (D) nasal turbinates. Gray area corresponds to levels below detection for the assay (E–H) Histopathological examination from lungs collected at 5 dpc from each group. (E) Mock-vaccinated group: Multifocally the alveolar septa are necrotic, collapsed, ruptured and thickened by hyaline membranes (arrowhead). Small to moderate numbers of macrophages, neutrophils, and lesser lymphocytes and plasma cells are infiltrating perivascular and peribronchial spaces, alveolar septa, and pleura (asterisks). (F) IGIP-H1att- and (G) H1caLen-vaccinated groups: Small multifocal clusters of lymphocytes and plasma cells are infiltrating peribronchial and perivascular spaces (asterisks). (H) Normal lung. (I–L) Immunohistochemistry against IAV antigens in lungs collected at 5 dpc. (H) Mock-vaccinated group: Moderate amount of IAV antigens is present as evidenced by the red staining. (J) IGIP-H1att, (K) H1caLen and (L) PBS groups. All representative pictures were taken at 20× magnification.