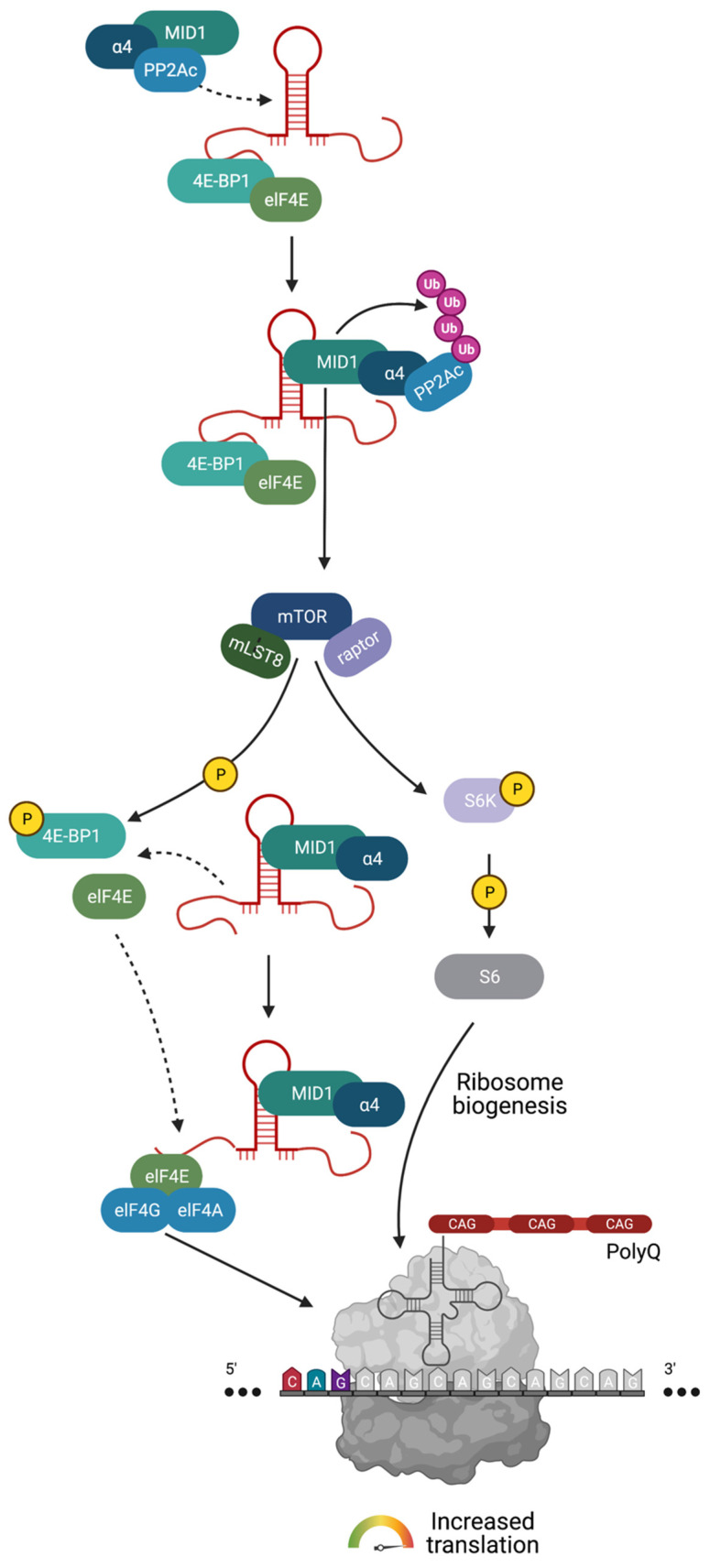
Figure 4.
The MID1 protein complex induces translation of HTT mRNA with expanded CAG repeats. MID1 (depicted in teal) attaches to HTT mRNA with expanded CAG repeats (hairpin depicted in red) and mediates the binding of translational regulators, including PP2A (depicted in blue). PP2A and its opposing kinase mTOR (depicted in dark blue) control the phospho-dependent activity of S6K (depicted in lilac) and 4E-BP1 (depicted in light green). 4E-BP1 is a negative regulator of translation that suppresses translation when bound to the 5′ end of an RNA. Its phosphorylation by mTOR leads to the detachment from RNA and the release of this translational block. At the same time, S6K gets activated by phosphorylation via mTOR. Phospho-activated S6K phosphorylates its target S6, which is a subunit of the ribosome. These two mTOR-dependent phosphorylation events promote ribosome assembly on the RNA and thus promote translation. Besides recruiting PP2A and S6K to the RNA hairpin, MID1 induces mTOR activity and simultaneously inhibits the activity of PP2A by inducing its proteasomal degradation. Thus, MID1 indirectly stimulates translation. Created with BioRender.com.