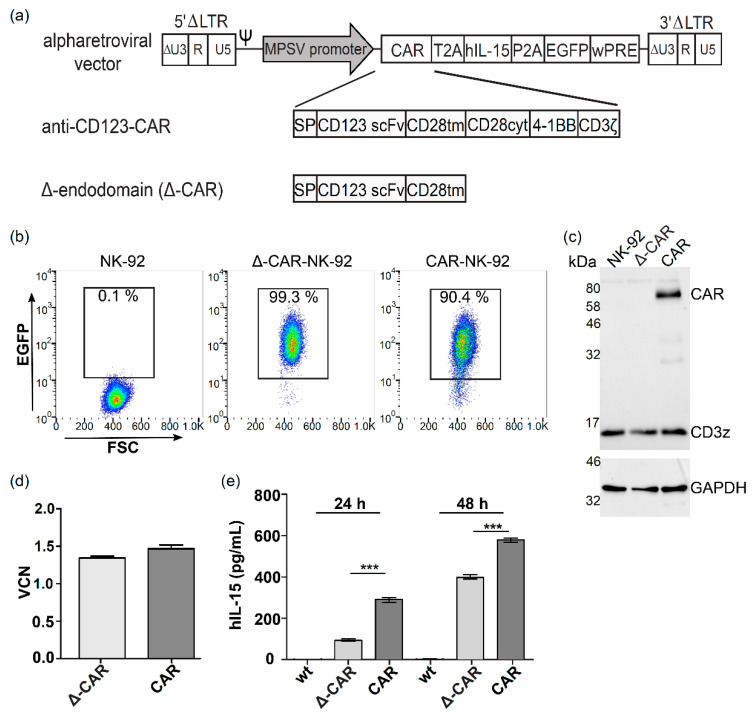
Figure 1.
Alpharetroviral vectors were used to generate anti-CD123-CAR-NK-92 cells, which were also engineered to secrete hIL-15. (a) Schematic of alpharetroviral vectors. The myeloproliferative sarcoma virus (MPSV) promoter was used to express all constructs, including the full-length anti-CD123-CAR, the truncated CAR (Δ-CAR), human interleukin-15 (hIL-15), and enhanced green fluorescent protein (EGFP). SP, signal peptide derived from the GM-CSF receptor; CD28tm, the transmembrane domain of CD28; CD28cyt, the cytoplasmic domain of CD28; LTR, long-terminal repeat (ΔU3, R and U5); Ψ, packaging signal; wPRE, woodchuck hepatitis virus post-transcriptional regulatory element. (b) Flow cytometric analyses showing enrichment of anti-CD123-CAR-NK-92 cells and Δ-CAR-NK-92 cells using the EGFP marker transgene to detect modified cells. Representative results from more than four experiments are shown. (c) The intact anti-CD123-CAR was detected via immunoblot experiments with an antibody directed against CD3ζ, which also detects the endogenous CD3ζ visualized as the marked band just below 17 kDa. An antibody to detect GAPDH was used as an additional control. Representative immunoblots from two to three experiments are shown. (d) Quantitative PCR showed similar vector copy numbers in CAR-NK-92 and Δ-CAR-NK-92 cells. Shown are mean values with standard deviations from experiments accomplished as technical triplicates. (e) hIL-15 secretion from unmodified NK-92 cells (wt), Δ-CAR-NK-92 cells or full-length CAR-NK-92 was quantified by ELISA. Experiments were accomplished twice in triplicate. Mean values with ranges are shown. Statistically significant differences are indicated by *** (p ≤ 0.001).