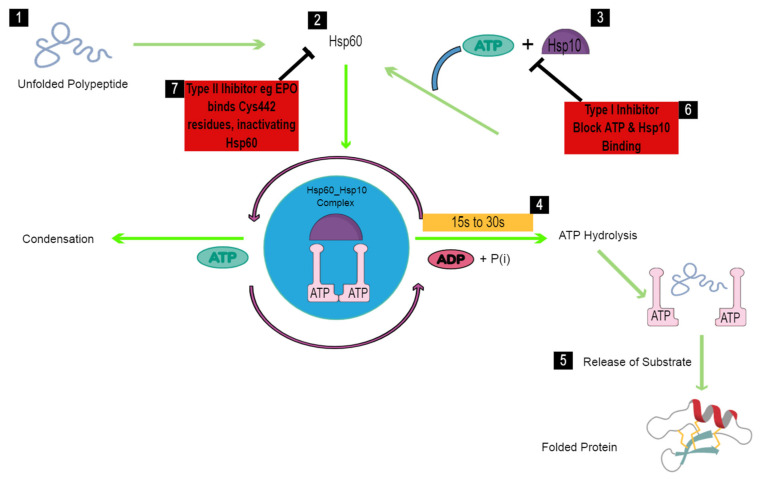
Figure 2.
Schematic diagram of the folding mechanism of the schistosomal Hsp60-Hsp10 complex. In the human host, schistosomes are exposed to extreme temperatures that result in unfolding of their proteins (1). Increased temperature conditions within the worm stimulate the expression of Hsp60, which binds the unfolded polypeptide (2). Hsp10 and ATP immediately bind to Hsp60, forming an Hsp60-Hsp10 complex that facilitates folding in an ATP-dependent manner (3). Protein folding is, therefore, expected to occur within 15–30 s prior to ATP hydrolysis (4), which leads to the release of the bound polypeptide (5), and thus, the worm regains functionality and survives. To block the chaperoning activity of Hsp60 in schistosomes, a type I Hsp60 inhibitor such as Mizoribine binds to Hsp60, blocking ATP and Hsp10 binding, and thus, preventing protein folding (6). A type II Hsp60 inhibitor, Epolactaene, binds the Cys442 residue, thus inactivating Hsp60 prior to binding of the unfolded polypeptide (7).