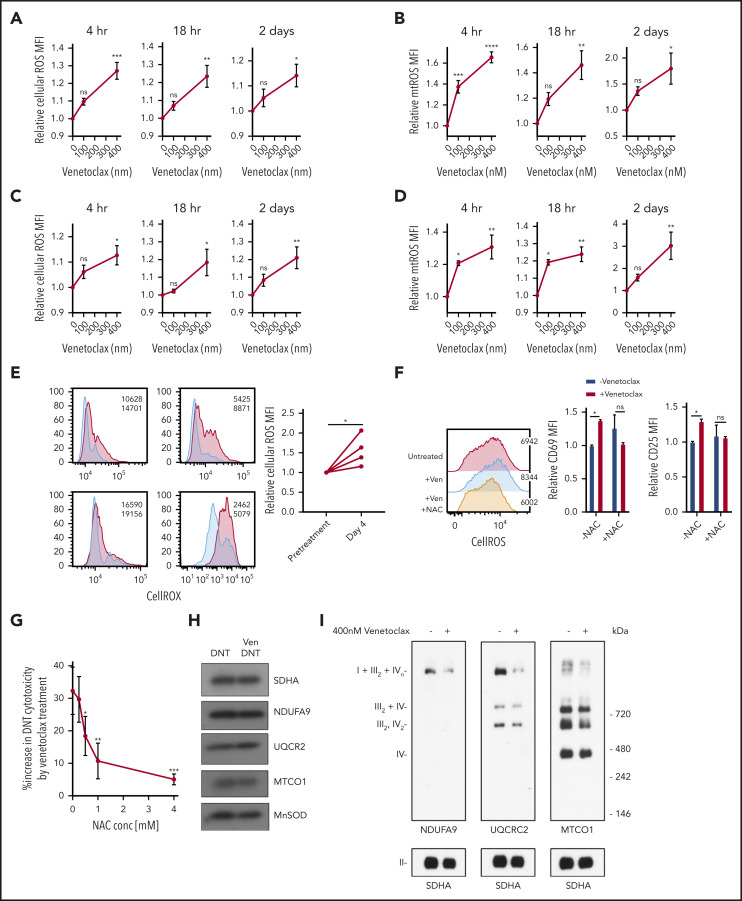
Figure 3.
Venetoclax increases ROS level to enhance T-cell effector function. (A-D) DNT (A-B) or Tconv cells (C-D) were treated with 0 nM, 100 nM, or 400 nM venetoclax for 4 hours, 18 hours, and 2 days. Cells were stained with CellROX (A,C) or MitoSOX (B,D). Median fluorescence intensity (MFI) of cellular or mitochondrial (mt) ROS was measured by using flow cytometry. Data represent the mean ± SEM of results from 4 different donor T cells. (E) Cellular ROS level in T cells obtained from 4 patients with AML before and during venetoclax and azacytidine treatment were determined by using flow cytometry. Flow histogram shows the CellROX staining for each patient’s T cells. Blue represents cellular ROS level in T cells from pretreatment samples and red represents those from day 4 of treatment. (F) DNTs treated with 400 nM venetoclax with or without 2 mM NAC for 18 hours. Flow histogram shows the cellular ROS level measured by using flow cytometry. MFI of CD25 and CD69 were measured by flow cytometry. Experiments were done in triplicate, and the data shown are representative of 2 independent experiments conducted by using DNTs from 2 donors. (G) DNTs treated with 400 nM venetoclax in the presence of increasing concentrations of NAC for 18 hours followed by coculture with OCI-AML2 for 2 hours at a 2:1 DNT:AML ratio. Experiments were done in triplicate, and data represent the percent increase in DNT-mediated cytotoxicity by venetoclax ± SD specific killing from 1 of 3 independent experiments done using DNTs from 3 different donors. (H) DNT cells were treated with 400 nM venetoclax for 18 hours. After treatment, mitochondria were isolated and levels of respiratory chain complex subunits were measured by sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and immunoblotting with antibodies against NDUFB8 (complex I), SDHA (complex II), UQCRC2 (complex III), and MTCO1 (complex IV). (I) Mitochondrial fractions were isolated after DNTs were treated with 400 nM venetoclax for 18 hours. Complex and respiratory chain supercomplex assembly were measured by blue native–polyacrylamide gel electrophoresis with antibodies against NDUFB8 (complex I), SDHA (complex II), UQCRC2 (complex III), and MTCO1 (complex IV). The results shown are representative of 3 independent experiments conducted by using DNTs from 3 different donors. Student t test or 1-way analysis of variance was used for statistics. *P < .05; **P < .01; ***P < .001; ****P < .0001.