Figure 3. Validation of Hoechst/PI cytotoxicity assay in leukemia cells.
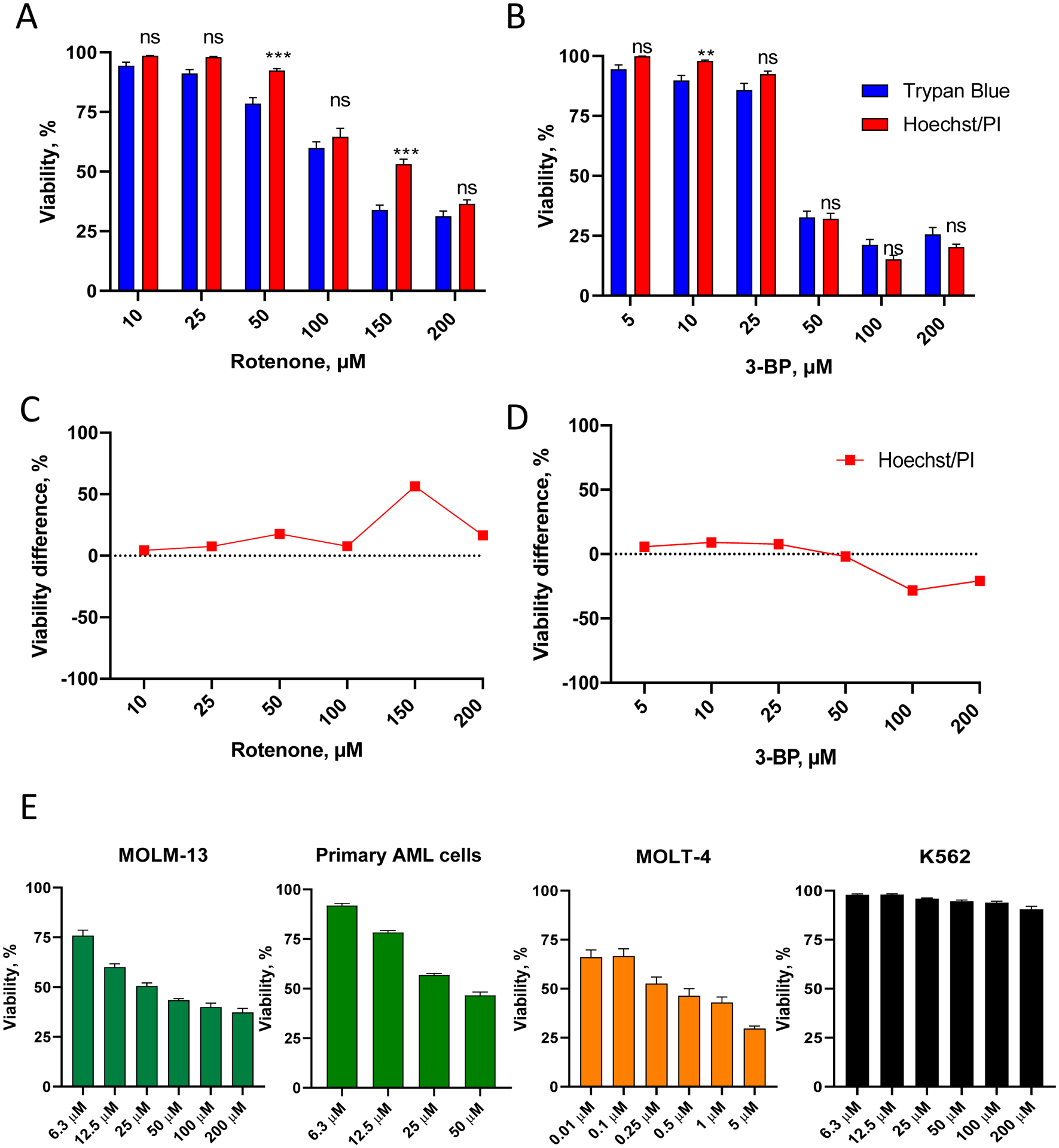
A-B. OCI-AML2 (AML) cells were treated with different concentrations of rotenone (A) or 3-bromopyruvate, 3-BP (B) in serum-free RPMI-1640 media for 24 h, then viability was determined. Shown is mean with SEM. C-D. Comparison of viability difference between Hoechst/PI assay vs. trypan blue staining (for the cells in A-B, see Supplementary Tables S3–4 for quantitation). Stars indicate statistical significance vs. trypan blue staining. ** p < 0.01, *** p < 0.001, ns – non-significant. Group comparison was done via t-test with correction for multiple hypothesis testing. E. MOLM-13 (AML), primary AML cells isolated from a patient, MOLT-4 (ALL), and K562 (CML) cells were treated with the indicated concentrations of rotenone in serum-free RPMI-1640 media for 24 h, prior to viability determination using Hoechst/PI staining. Shown is mean with SEM. Three independent biological replicates were performed.
