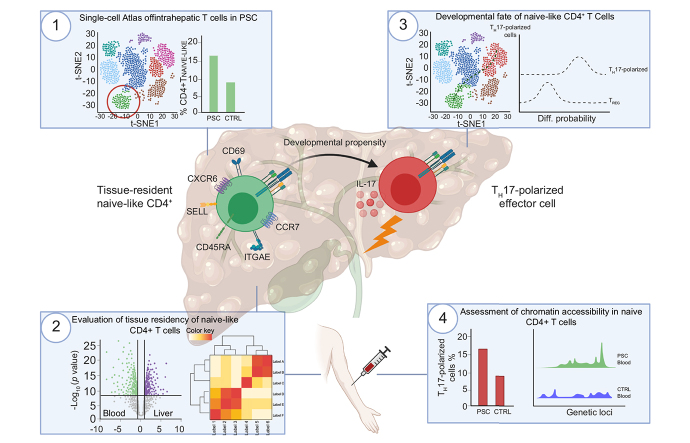
Abstract
Background & Aims
Little is known about the composition of intrahepatic immune cells and their contribution to the pathogenesis of primary sclerosing cholangitis (PSC). Herein, we aimed to create an atlas of intrahepatic T cells and thereby perform an in-depth characterization of T cells in inflamed human liver.
Methods
Different single-cell RNA sequencing methods were combined with in silico analyses on intrahepatic and peripheral T cells from patients with PSC (n = 11) and healthy donors (HDs, n = 4). Multi-parameter flow cytometry and functional in vitro experiments were conducted on samples from patients with PSC (n = 24), controls with other liver diseases and HDs.
Results
We identified a population of intrahepatic naive-like CD4+ T cells, which was present in all liver diseases tested, but particularly expanded in PSC. This population had a transcriptome and T cell receptor repertoire similar to circulating naive T cells but expressed a set of genes associated with tissue residency. Their periductal location supported the concept of tissue-resident naive-like T cells in livers of patients with PSC. Trajectory inference suggested that these cells had the developmental propensity to acquire a T helper 17 (TH17) polarization state. Functional and chromatin accessibility experiments revealed that circulating naive T cells in patients with PSC were predisposed to polarize towards TH17 cells.
Conclusion
We report the first atlas of intrahepatic T cells in PSC, which led to the identification of a previously unrecognized population of tissue-resident naive-like T cells in the inflamed human liver and to the finding that naive CD4+ T cells in PSC harbour the propensity to develop into TH17 cells.
Lay summary
The composition of intrahepatic immune cells in primary sclerosing cholangitis (PSC) and their contribution to disease pathogenesis is widely unknown. We analysed intrahepatic T cells and identified a previously uncharacterized population of liver-resident CD4+ T cells which are expanded in the livers of patients with PSC compared to healthy liver tissue and other liver diseases. These cells are likely to contribute to the pathogenesis of PSC and could be targeted in novel therapeutic approaches.
Keywords: Primary Sclerosing Cholangitis, Immune-mediated liver disease, Single-cell sequencing, Atlas, T cells, Naive T cells, TH17 cells, Tissue residency
Graphical abstract
Highlights
-
•
First single-cell atlas of intrahepatic T cell landscape in PSC.
-
•
Identification of tissue-resident naive-like CD4+ T cells in human liver which are expanded in livers of patients with PSC.
-
•
Trajectory inference suggested a developmental propensity of these cells to acquire a TH17 polarization state.
-
•
Functional experiments, using circulating naive CD4+ T cells, support the inferred propensity to acquire a TH17 polarization state.
-
•
Chromatin accessibility studies revealed imprinting of naive CD4+ T cells towards effector function in PSC.
Introduction
Primary sclerosing cholangitis (PSC) is a prime example of an immune-mediated liver disease, although its pathogenesis remains poorly understood. PSC is a progressive fibro-obliterative disease of the intra- and/or extrahepatic bile ducts that lacks any effective therapy and often progresses to cirrhosis, liver transplantation or death. PSC is strongly associated with inflammatory bowel disease (IBD), and it confers a substantially increased risk of both hepatobiliary and colorectal malignancies.[1], [2], [3] The gut-liver axis is a conundrum in PSC and therefore both intestinal microbiota and various immune cells have been studied. The gut microbiota were consistently found to show less diversity in patients with PSC, which was recently shown to hold true for the biliary microbiota as well.[4], [5], [6]
Genome-wide association studies (GWAS) implicated T cells in the pathogenesis of PSC[7], [8], [9] and phenotypic changes of T cells were detected in livers and peripheral blood of patients with PSC.
Recent studies highlighted the power of single-cell transcriptomics to unravel the heterogeneity of liver-parenchymal cells,10,11 whereas little is known about the composition and function of immune cells isolated from inflamed human livers. Although intrahepatic immune cells mostly comprise innate cells, liver-resident T cells have emerged as central mediators of liver injury.[12], [13], [14] Tissue residency is a feature assigned to memory T cells, which is established during the immune response in non-lymphoid tissues.15 In contrast to memory T cells, naive T cells usually circulate between blood and lymph nodes and whether they are able to reside in non-lymphoid tissues such as the liver has never been substantiated in humans.16 Few studies have analysed the intrahepatic T cell compartment in PSC,[17], [18], [19], [20], [21] mostly due to the rarity of the disease and the limited availability of tissue specimens.
Compiling a single-cell atlas in PSC could substantially improve our understanding of the role of tissue-resident T cells in the pathophysiology of PSC and immune-mediated liver diseases in general.
Herein, we provide the first single-cell atlas of intrahepatic T cells in PSC, thereby unravelling the heterogeneity of intrahepatic T cells as important drivers of immune-mediated liver disease.
We identified a previously unrecognized population of intrahepatic naive-like CD4+ T cells, which display a gene signature accounting for tissue residency and a developmental propensity towards an effector fate in livers of patients with PSC. We extended these findings to circulating bona fide naive CD4+ T cells, which show a higher capacity to acquire T helper 17 (TH17)-associated effector functions, as well as a corresponding epigenetic profile, in patients with PSC compared to healthy controls.
These data render naive CD4+ T cells likely contributors to the pathogenesis of PSC and point towards a so far underestimated role of naive T cells in the development of immune-mediated liver diseases, thereby offering potential novel treatment targets.
Materials and methods
For details regarding the materials and methods used, please refer to the CTAT table and supplementary information.
Results
Single-cell atlas of intrahepatic T cells reveals a population of naive-like CD4+ T cells in livers of patients with PSC
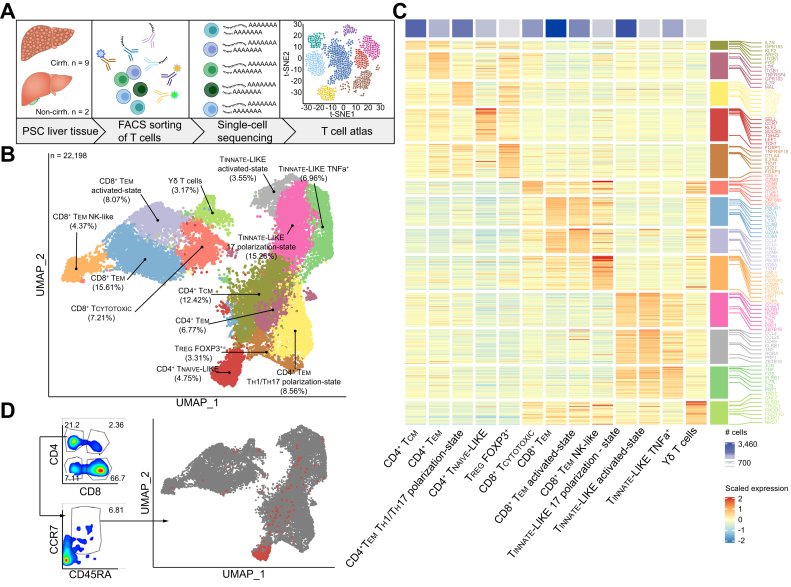
We performed single-cell RNA sequencing (scRNA-Seq) of intrahepatic T cells obtained from 11 patients with PSC. In addition, for 6 of these samples we created protein surface marker libraries using cellular indexing of transcriptomes and epitopes by sequencing (CITE-Seq). Intrahepatic T cells were isolated and FACS-sorted from either explanted livers (n = 9) or non-cirrhotic livers (n = 2) from patients with PSC (Fig. 1A and Fig. S1A and Table S1). In total, 22,198 sequenced intrahepatic T cells were included.
Fig. 1.
Single-cell atlas of intrahepatic T cells reveals a population of naive-like CD4+ T cells in PSC.
(A) Graphical abstract of the workflow on fresh and cryopreserved cells deriving from livers of patients with PSC. (B) Atlas of 22,196 intrahepatic T cells from patients with PSC (n = 11), showing 13 distinct clusters. (C) Cluster-Heatmap highlighting signatures of DEGs for each cluster of the atlas. (D) Hierarchical gated CD4+ T cells, using the ADT for CCR7 and CD45RA, are shown within the atlas, highlighting the naive-like cluster. See also Figs. S1, 6-8 and Table S1. ADT, antibody-derived tags; DEGs, differentially expressed genes; PSC, primary sclerosing cholangitis; TCM, central memory cell; TEM, effector memory cell; TCYTOTOXIC, cytotoxic T lymphocyte; TREG, regulatory T cell; TEM TH1/TH17-state, effector memory cell with a TH1 and TH17 polarization state; UMAP, uniform manifold approximation and projection. Graphical abstract was created with BioRender.com.
Within these cells, we identified 13 distinct clusters of intrahepatic T cells (Fig. 1B-C and Fig. S1B) by differentially expressed genes and protein expression analysed via CITE-Seq antibodies (antibody-derived tags). Among these 13 clusters, 4 showed none or random expression of CD4 and CD8 and shared the expression of innate-like genes (Fig. S1B). One of these clusters was assigned to T-gd cells, expressing TRDC, TRGC1, and genes associated with cytotoxicity, including GNLY, and innate immune response genes such as FCER1G. The remaining 3 clusters were characterized by high expression of KLRB1 and CCR6, and surface markers CCR6 and CD56, which are known hallmarks of mucosal-associated invariant T cells.22 These 3 clusters of innate-like T cells exhibited distinct functional phenotypes: (i) TH17-associated properties (TINNATE-LIKE 17-state), e.g. by expressing the transcription factor RORA; (ii) a pro-inflammatory cellular state (TINNATE-LIKE TNFa+), characterized by expressing high levels of TNF; (iii) an activated cellular state (TINNATE-LIKE activated-state), by expression of chemokines CCL20, CCL4 and CD69 (Fig. 1C and Fig. S1B).
Besides the aforementioned ‘non-classical’ T cell clusters, we also identified 4 clusters of CD8+ T cells and 5 clusters of CD4+ T cells. Among the 4 CD8+ T cell clusters, we identified effector memory cells (CD8+ TEM), characterized by low expression of CCR7 and low expression of granzymes; CD8+ T cells with an activated cellular state (CD8+ TEM activated-state), characterized by the expression of effector molecules such as IFNG, CCL4, CCL5, and GZMK; cytotoxic CD8+ T cells (CD8+ TCYTOTOXIC) expressing cytotoxicity-related genes such as GNLY, GZMB, CD63, and tissue residency gene ZNF683 (HOBIT); and CD8+ T cells, with features of natural killer (NK) cells, expressing genes such as NKG7, KLRD1, FGFBP2, and FCGR3A, which we refer to as ‘CD8+ TEM NK-like’ (Fig. 1C and Fig. S1B).
The CD4+ T cell population consisted of effector memory (CD4+ TEM) and central memory (CD4+ TCM) cell clusters, characterized by the typical expression of signature genes (MAL, IL7R, LTB, TNFRSF4) and surface markers (i.e. IL-7R, CD45RO and CCR7). Furthermore, we identified FOXP3+ regulatory T cells (TREG) by the expression of signature genes IL2RA and FOXP3 as well as co-inhibitory molecules TIGIT and CTLA4, and the expression of surface markers such as CD25 (Fig. 1C and Fig. S1B). A cluster of CD4+ TEM cells with properties of both T helper 1 (TH1) and TH17 (CCR6, KLRB1, MAF and CXCR6) polarization states was also identified. We further elucidated this cluster by analysing expression of signature genes for TH1 (IFNG, TNF, TBX21, CXCR3 and IL12RB1) as well as TH17 cells (IL17A, RORC, RORA, IL23R and CCR6) and thereby confirmed the presence of cells with both polarization states (Fig. 1C and Fig. S1B-D). Therefore, we termed the cluster “CD4+ effector memory cells with a TH1 and TH17 polarization state” (CD4+ TEM TH1/TH17 polarization state). Surprisingly, 1 cluster of intrahepatic CD4+ T cells was characterized by a gene signature including SELL, CCR7, TSHZ2, LEF1, and TCF7 (Fig. 1B-C), as well as co-expression of CD45RA and CCR7 (Fig. 1D and Fig. S1B), which are typical features of naive T cells.[23], [24], [25], [26], [27], [28] Single-cell TCR sequencing (scTCR-Seq) of these CD4+ T cells with a naive phenotype revealed a high clonotype-to-cell ratio, showing that these cells did not undergo clonal expansion and thereby confirming their naive phenotype (Fig. S1E).
Intrahepatic naive-like CD4+ T cells are present in liver diseases of different aetiologies and particularly expanded in patients with PSC
Naive CD4+ T cells usually circulate between lymphoid tissue and peripheral blood. We therefore decided to explore whether the presence of this population within the liver was a unique feature of PSC, or a feature common to other inflammatory liver diseases of different aetiologies (Fig. 2A).
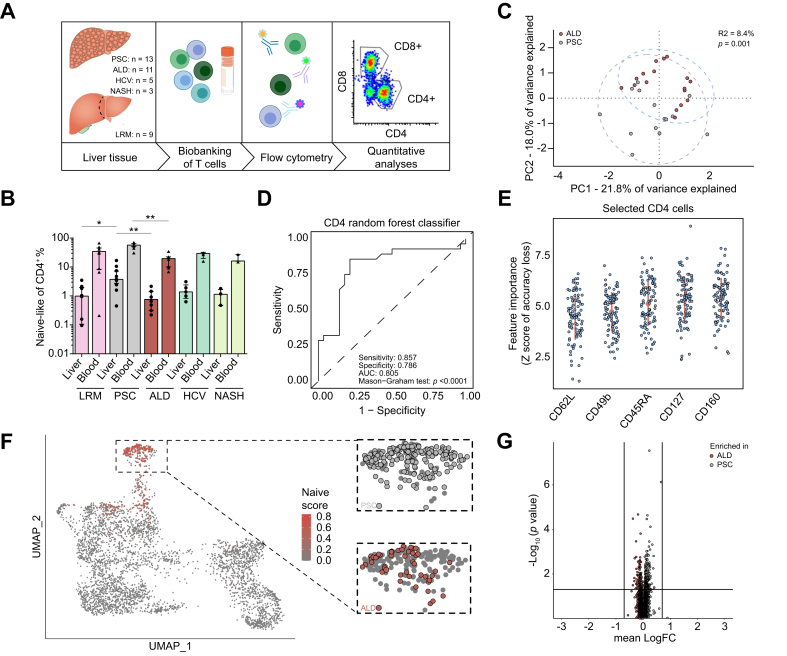
Fig. 2.
Intrahepatic naive-like CD4+ T cells are present in inflammatory liver diseases and expanded in patients with PSC.
(A) Graphical abstract of the workflow on cryopreserved cells derived from patients with liver diseases of different aetiologies. (B) Frequencies of naive and naive-like CD4+ T cells from blood and liver, respectively, of the same patients determined by classical hierarchical gating for CCR7 and CD45RA. PSC showed the highest frequencies in both liver (PSC vs. ALD, p = 0.0039; PSC vs. LRM, p = 0.0205) and blood (PSC vs. ALD, p = 0.0039). Data in (B) is presented as median (IQR). Statistical significance within blood or liver was assessed by Kruskal-Wallis test (Liver: p = 0.0018; Blood: p = 0.0049). (C) PCA of multi-parameter flow cytometry data of intrahepatic CD4+ T cells from late-stage PSC (grey) and ALD (red). Permutational analysis of variance on Euclidean distance was performed to determine statistical differences between the groups (p = 0.001; R2 = 8.4%). (D) Random forest classifier for intrahepatic CD4+ T cells of PSC and ALD. Discrimination was assessed by AUC, specificity and sensitivity. Statistical significance of the classifier performance was tested by Mason’s and Graham’s non-parametric test. p <0.05 was considered statistically significant. (E) Populations of intrahepatic CD4+ T cells identified to best discriminate between PSC and ALD: identified by iteratively removing the features statistically proven to be less relevant than random samples generated by permutation of the original variable values. (F) UMAP projection of 4,072 intrahepatic T cells of late-stage PSC and ALD. Projection of naive-like DEG core signature, extracted from the atlas, is shown. Cells with matching transcriptomes are highlighted in red. Contribution of underlying disease to the cluster of naive-like CD4+ T cells is shown in the respective colour. (G) Volcano plot of DEG between intrahepatic naive-like CD4+ T cells from late-stage PSC and ALD, indicating similarity of gene expression. Lines indicate cut-off of statistical significance (p <0.05) and logarithmic fold-change of expression (|logFC| >0.7). See also Fig. S2 and Tables S2-3. ALD, alcohol-related liver disease; DEG, differentially expressed gene; LRM, liver resection margin; NASH, non-alcoholic steatohepatitis; PCA, principle component analysis; PSC, primary sclerosing cholangitis; UMAP, uniform manifold approximation and projection. Graphical abstract was created with BioRender.com.
We confirmed the presence of these cells in liver tissue across different liver diseases (PSC, n = 16; non-cirrhotic liver resection margin [LRM] n = 10; chronic HCV infection, n = 5; non-alcoholic steatohepatitis [NASH] n = 3; alcohol-related liver disease, ALD, n = 16, Table S2) using multi-parameter flow cytometry. However, the highest abundance was seen in livers of patients with PSC (n = 16) (p = 0.0018, Fig. 2B), which was also observed in peripheral blood of the same patients (n = 8) (p = 0.0049, Fig. 2B), both irrespective of associated IBD (Fig. S2A-B). When correlating the abundance of these cells within both compartments, we observed a significant correlation (p <0.0001, r=0.7972, Fig. S2C). We next aimed to validate the finding of enriched naive-like CD4+ T cells in PSC using an unsupervised approach. First, we performed a principal component analysis (PCA) considering all intrahepatic CD4+ T cell populations, e.g. naive, effector and memory, and found significant overall differences between patients with PSC and ALD (p = 0.001; R2 = 8.4%) (Fig. 2C). Second, to determine which T cell populations accounted for the differences between PSC and ALD, we utilized random forest machine learning, using 24 markers defining CD4+ T cell populations and subpopulations (Table S3). We obtained an accurate and statistically significant classification performance that distinguished between patients with PSC and ALD (p <0.0001; AUC = 0.805; sensitivity = 0.857; specificity = 0.786) (Fig. 2D). The obtained classifier identified 5 CD4+ T cell surface markers, including CD45RA and CD62L, which are classical markers of naive T cells27 (Fig. 2E and Fig. S2D).
We next asked whether naive-like CD4+ T cells bear qualitative differences among liver diseases. Therefore, we performed scRNA-Seq on T cells isolated from the livers of patients with ALD (n = 3) and integrated the data with scRNA-Seq data from patients with PSC (n = 5) extracted from the atlas. First, we confirmed that the intrahepatic naive-like CD4+ T cells form a distinct cluster (Fig. 2F and Fig. S2E-F). Second, we found that the gene expression profiles of intrahepatic naive-like CD4+ T cells were similar between PSC and ALD (Fig. 2G). These results show that intrahepatic naive-like CD4+ T cells are particularly enriched in livers of patients with PSC but have a similar transcriptional profile across different liver diseases.
Intrahepatic naive-like CD4+ T cells bear evidence of tissue residency
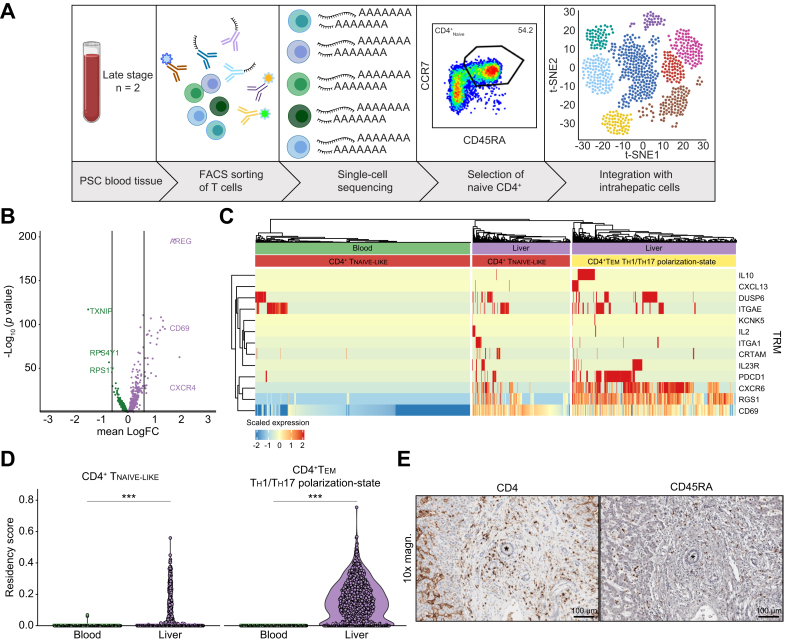
Next, we wondered whether these intrahepatic naive-like CD4+ T cells are tissue-resident or merely circulating through the liver. To address this question, we compared bona fide naive CD4+ T cells from peripheral blood and intrahepatic naive-like CD4+ T cells from the same PSC patients (n = 2, Table S4) by CITE-Seq (Fig. 3A). We compared the transcriptomic profiles of peripheral naive CD4+ T cells and intrahepatic naive-like CD4+ T cells after selecting them in the dataset according to their feature gene expression profiles (Fig. S3A). Despite an overall similarity, we observed selective expression of CD69, a hallmark of tissue residency, and CXCR4, which is associated with T cell homing towards the biliary epithelium,29 in the intrahepatic naive-like CD4+ T cells (Fig. 3B). To specifically evaluate the expression of tissue residency genes in naive-like CD4+ T cells, we tested the accordance of these cells to literature-based gene profiles of CD69+ tissue-resident T cells.30 The expression of a signature associated with tissue residency was seen in liver-derived naive-like CD4+ T cells (Fig. 3C). We next quantified the accordance to this signature, which was significantly higher in the intrahepatic cells compared to the circulatory cells from blood (p <0.001, Fig. 3D). The population of CD4+ TEM TH1/TH17 polarization state from the same dataset was selected as a positive control for tissue residency. Of note, quantitative classification revealed that 38.36% of intrahepatic naive-like CD4+ T cells had a tissue-resident phenotype (Fig. S3B).
Fig. 3.
Intrahepatic naive-like CD4+ T cells bear a tissue-resident phenotype.
(A) Graphical abstract of the workflow on cryopreserved cells deriving from blood and corresponding livers of patients with PSC. (B) Volcano plot of DEG between intrahepatic naive-like CD4+ T cells and bona fide naive CD4+ T cells from the same individuals with PSC showing significant expression of genes accounting for tissue residency. Lines indicate logarithmic fold-change of expression (|logFC|>0.6). (C) Heatmap visualizing gene expression of naive CD4+ T cells coloured by cell origin (green: blood; purple: liver). Expression of a literature-based gene signature for tissue residency is shown. CD4+ TEM TH1/TH17 polarization state from the same dataset was used as positive control. (D) Violin plot quantifying accordance with literature-based residency-score including CD69 (p <0.001 as determined by Wilcoxon signed-rank test). CD4+ TEM TH1/TH17 polarization state from the same dataset was used as positive control (p <0.001 as determined by Wilcoxon signed-rank test). (E) Immunohistochemistry staining of CD45RA and CD4, respectively, in a liver with PSC. Bile ducts are indicated by asterisks and scale bars indicating 100 μm. See also Fig. S3 and Table S4. DEG, differentially expressed gene; PSC, primary sclerosing cholangitis; TEM TH1/TH17-state, effector memory cell with a TH1 and TH17 polarization state; TH1, T helper 1; TH17, T helper 17; TRM, tissue-residency marker. Graphical abstract was created with BioRender.com.
Finally, to confirm the presence of naive-like CD4+ T cells within livers, we performed immunohistochemistry on consecutive slides from liver samples included within our scRNA-Seq dataset. We found CD4+ and CD45RA+ T cells to be located within the portal tracts, which is the site of periductular inflammation and fibrosis in PSC1,2 (Fig. 3E). All these data combined show that a significant fraction of intrahepatic naive-like CD4+ T cells, despite overall similarity to the circulatory naive CD4+ T cells from peripheral blood, had acquired a tissue-resident phenotype in PSC. These data contribute to the concept of tissue-resident naive T cells in non-lymphoid tissues.
Peripheral naive and intrahepatic naive-like CD4+ T cells show a propensity to develop towards effector cells with a TH17 polarization state in patients with PSC
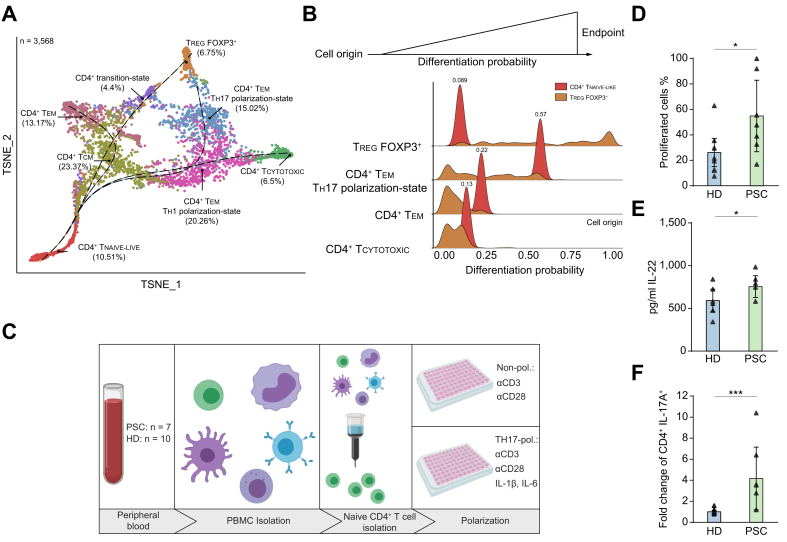
To gain functional insight into the population of naive-like CD4+ T cells resident in livers of patients with PSC, we assessed the developmental fate of these cells by applying trajectory analysis to the transcriptomic data. Therefore, we first extracted the CD4+ T cells (n = 6, Table S5) from our T cell atlas via CITE-Seq antibodies and second re-clustered these cells. Hereby, we identified 8 distinct clusters of CD4+ T cells including CD4+ TEM, CD4+ TCM, Foxp3+ TREG and naive-like cell populations like within the atlas (Fig. S4A-B). We applied Slingshot31 to this dataset, defining the liver-resident naive-like CD4+ T cells as the developmental origin for this trajectory analysis. Four major developmental trajectories were observed (Fig. 4A). Next, Palantir’s algorithm,32 a Markov chain-based approach for estimating differentiation probabilities, was used to determine the probabilities of the naive-like CD4+ T cells developing into defined subsets. We used the respective endpoints of each trajectory, i.e. CD4+ TEM TH17-state, CD4+ TEM, CD4+ TCYTOTOXIC and Foxp3+ TREG, as these subsets. Interestingly, we observed that the naive-like CD4+ T cells showed by far the highest probability (0.57) of differentiating towards the TH17 polarization state compared to all other endpoints (Fig. 4B). We used TREG as negative control since their differentiation potential was determined to be low. Interestingly, we detected a significant positive correlation between the frequencies of intrahepatic naive-like CD4+ T cells and TH17-polarized cells within the same livers from our flow cytometry dataset (r = 0.4638, p = 0.0195, Fig. S4C).
Fig. 4.
Propensity of naive-like CD4+ T cells to develop towards effector cells with a TH17 polarization state in patients with PSC.
(A) Diffusion map based t-SNE projection of 3,568 extracted intrahepatic CD4+ T cells from the CITE-Seq dataset of patients with PSC (n = 6, freshly processed samples), showing 8 distinct clusters. Slingshot was applied with naive-like CD4+ T cells defined as starting point. (B) Palantir analysis of the data from (A). Mean probabilities of naive-like CD4+ T cells to differentiate into CD4+ effector memory cells with a TH17 polarization state (CD4+ TEM TH17 polarization-state), CD4+ effector memory (CD4+ TEM), cytotoxic CD4+ (CD4+ TCYTOTOXIC) T cells and regulatory T cells (TREG) were determined. TREG were included as negative control. (C), Graphical abstract of the workflow of in vitro experiments on freshly processed blood samples. (D,E) In vitro culture of blood-deriving naive-like CD4+ T cells under non-polarizing conditions for 7 days (PSC: n = 7; HD: n = 10). (D) Proliferation was determined by detection of CellTrace Violet (Mann-Whitney U; p = 0.0231) and (E) secretion of IL-22 was analysed by multiplex ELISA (Mann-Whitney U; p = 0.0429). (F) In vitro culture of blood-deriving naive-like CD4+ T cells under TH17-polarizing conditions for 12 days (PSC: n = 7; HD: n = 10). Fold change of frequencies of CD4+IL-17A+ cells normalized to the respective controls of each experiment (n = 4) (Mann-Whitney U; p <0.001). Data in (D,E,F) is presented as median (IQR). See also Fig. S4 and Table S5. CITE-Seq, cellular indexing of transcriptomes and epitopes by sequencing; HD, healthy donor; PBMC, peripheral blood mononuclear cell; PSC, primary sclerosing cholangitis; TH17, T helper 17; t-SNE, t-distributed stochastic neighbour embedding. Graphical abstract was created with BioRender.com.
It was not possible to isolate naive-like CD4+ T cells from the liver in sufficient numbers to perform functional in vitro assays. We therefore conducted functional experiments using naive CD4+ T cells isolated from peripheral blood of patients with PSC (n = 8) and healthy donors (HDs; n = 10) (Fig. 4C and Table S5). Of note, naive CD4+ T cells from the peripheral blood closely resembled the tissue-resident naive-like CD4+ T cells, as shown by gene expression analysis (Fig. 3B and Fig. S3A).
When cultured under non-polarizing conditions (aCD3/aCD28 only) for 7 days, naive CD4+ T cells isolated from the peripheral blood of patients with PSC showed a higher rate of proliferation (p = 0.0231) (Fig. 4D), and secreted higher amounts of IL-22, a cytokine characteristic of TH17 cells33,34 (p = 0.0429) (Fig. 4E). We next assessed the capacity of naive CD4+ T cells from peripheral blood to develop into TH17 cells under polarizing conditions. We detected an increased frequency of IL-17A-producing CD4+ T cells in PSC compared to HDs after 12 days of culture (p <0.001) (Fig. 4F and Fig. S4E-F). In contrast, the frequencies of IFNg-producing CD4+ T cells were similar between patients with PSC and controls, as was the secretion of IFNg into the supernatant (Fig. S4G-I).
Peripheral naive CD4+ T cells in PSC show evidence of epigenetic imprinting towards effector function
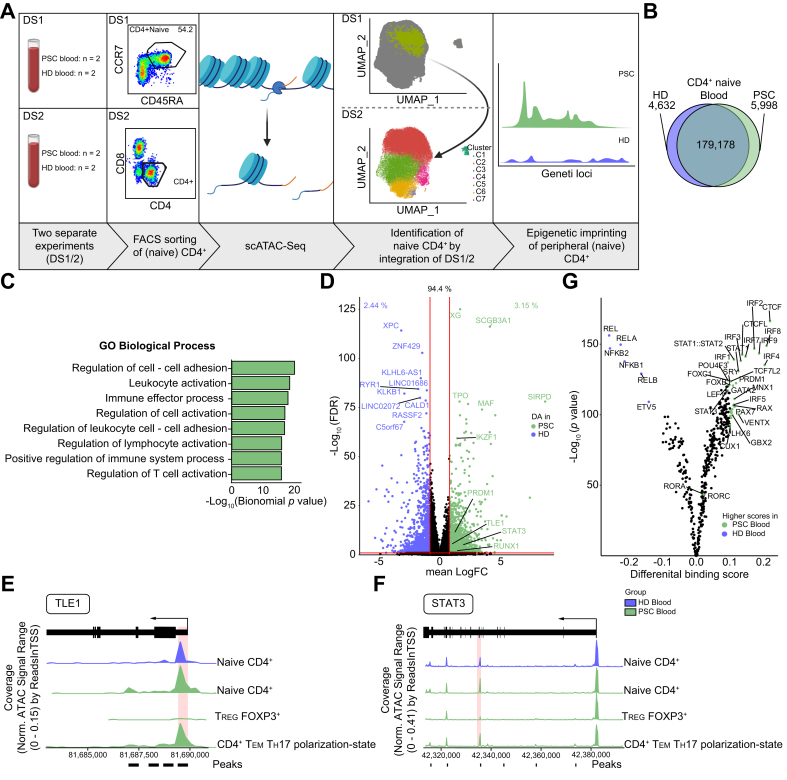
Considering the in silico and in vitro data, we wondered whether there is an epigenetic predisposition within PSC-derived naive T cells to acquire, particularly, TH17-polarized effector functions. Therefore, we performed single-cell assays for transposase accessible chromatin (scATAC-Seq) on FACS-sorted naive CD4+ T cells from peripheral blood of patients with PSC (n = 2) and HDs (n = 2), constituting dataset 1 (DS1). In addition, we included CD4+ T cells from blood of patients with PSC (n = 2) and HDs (n = 2), constituting dataset 2 (DS2) (Table S6). First, we integrated both datasets and used the first dataset to identify the naive CD4+ T cells from the second dataset (Fig. 5A and Fig. S5A-C). Second, we compared naive CD4+ T cells from patients with PSC to HDs and found the overall accessible chromatin regions to be similar between the 2 groups (n = 179,178; Fig. 5B). However, differentially accessible regions were detected, with 5,998 differential accessible regions in PSC. To gain an overview of these regions, we applied the Genomic Regions Enrichment of Annotations Tool (GREAT35), which assigned processes of cellular activation and adhesion to the chromatin regions of increased accessibility in PSC (Fig. 5C).
Fig. 5.
Epigenetic predisposition of naive CD4+ T cells in PSC.
(A) Graphical abstract of the workflow of scATAC-Seq experiments. Peripheral naive CD4+ and total CD4+ T cells from patients with PSC (n = 4) and HDs (n = 4) were included. Cryopreserved cells were used for these experiments. (B) Venn diagram of shared and differential chromatin accessibility between naive CD4+ T cells from blood of patients with PSC (n = 4) and HDs (n = 4). (C) Volcano plot highlighting mapped regions of shared and significantly different chromatin accessibility between naive CD4+ T cells from blood of patients with PSC (n = 4) and HDs (n = 4). (D) Significantly enriched gene ontology terms regarding biological processes obtained by GREAT analysis of the 5,998 differentially accessible chromatin regions of naive CD4+ T cells between patients with PSC and HDs. (E-F) Coverage plots for selected markers associated with TH17 cell polarization, i.e. TLE1 (E) and STAT3 (F) showing increased chromatin accessibility within naive CD4+ T cells from PSC blood compared to HD blood. Chromatin regions of significantly increased accessibility in PSC are indicated by marks below the coverage plots. Peaks with the lowest FDR/highest Log2FC are highlighted with red boxes. TREG and CD4+ TEM TH17-polarization state were included as a control for visualization. (F) TOBIAS analysis showing differential TFBS accessibility between blood-derived naive CD4+ T cells from patients with PSC (n = 4) and HDs (n = 4). Accessible TFBS of IRFs, STATs, PRDM1 and RORs indicating cellular activation. See also Fig. S5 and Tables S6-7. FDR, false discovery rate; HD, healthy donor; PSC, primary sclerosing cholangitis; TFBS, transcription factor binding site; TH17, T helper 17; TEM TH17 polarization-state, effector memory cells with a TH17 polarization state; TREG, regulatory T cells. Graphical abstract was created with BioRender.com.
Interestingly, screening of the regions of increased accessibility in PSC revealed several genes associated with T cell activation and TH17-polarization, e.g. STAT3, TLE1, RUNX1, as well as MAF (Fig. 5D). Several chromatin regions of STAT3, as well as the promotor region of TLE1, were found to be more accessible within naive CD4+ T cells from patients with PSC (Fig. 5E-F and Table S7). In order to gain additional functional insight, we utilized Transcription factor Occupancy prediction By Investigation of ATAC-seq Signal (TOBIAS).36 This allowed us to detect open transcription factor binding sites (TFBS) within naive CD4+ T cells and compare them between patients with PSC and HDs. We observed significantly more accessible TFBS of interferon-regulatory factors, STATs and PRDM1 (Blimp1), indicating overall cellular activation in PSC. Interestingly, RORA and RORC, which are associated with TH17-polarization, were detectable among the transcription factors with more accessible TFBSs, albeit with a rather low differential binding score.
In summary, these data suggest that the propensity of PSC-derived circulatory naive CD4+ T cells to develop towards effector phenotypes, particularly a TH17 polarization state, is in part epigenetically imprinted.
Discussion
Understanding autoimmune diseases requires the analysis of affected tissue, which harbours infiltrating and tissue-resident immune cells as well as their target cells, and thus displays a disease specific microenvironment. However, few studies have analysed the intrahepatic T cell compartment in PSC,[17], [18], [19], [20], [21] mostly due to the rarity of the disease and the limited availability of tissue specimens.
Deciphering the cellular composition of inflamed organs may reveal novel treatment targets. By combining complementary unbiased approaches such as scRNA-Seq, scATAC-Seq, scTCR-Seq and CITE-Seq with multi-parameter flow cytometry, we addressed this unmet need in PSC in unprecedented depth. With this, we present a comprehensive landscape of intrahepatic T cells in PSC, which we hope will be a valuable resource for the field.
We report on a previously unrecognized population of naive-like CD4+ T cells which are expanded in the livers of patients with PSC. Although the current dogma affirms naive T cells being primed in lymphoid tissues and polarized towards effector cells depending on the microenvironment,16 the observation of T cells with a naive phenotype has previously been described in livers of patients with autoimmune liver disease19 and healthy individuals,37 as well as other tissues such as the colon.38 However, detailed analysis of this cell subset was not performed in these studies.
A hallmark of naive T cells is their high expression of CCR7 and CD62L,27,28 which were highly expressed by the naive-like CD4+ T cells. The expression of the ligands to these molecules, CCL21 and MAdCAM-1, respectively, has been reported within livers of patients with PSC.19,[39], [40], [41] Of note, the expression of other ligands of MAdCAM-1, e.g. integrins alpha-4 and beta-7, which were supposed to facilitate aberrant liver homing of gut-primed cells,17 was not detectable within these cells.
Interestingly, the identified population of intrahepatic naive-like CD4+ T cells expressed CD69 and CXCR4, genes required for activation and homing towards the biliary epithelium within the liver.29 These cells also expressed a gene signature of tissue residency, and thus could represent tissue-resident T cells.30 Naive T cells being tissue-resident in non-lymphoid tissues, such as the liver, have been a matter of debate and a study of the developmental trajectory of these cells within non-lymphoid tissue has never been performed.27
In this study, we report that intrahepatic naive-like CD4+ T cells are prone to develop a TH17 rather than a Foxp3+ TREG polarization state in patients with PSC. Of note, intrahepatic naive-like CD4+ T cells were present in all liver diseases analysed, but their abundance was highest in patients with PSC. TH17 cells have been implicated in the pathogenesis and disease progression of PSC and other inflammatory liver diseases, such as HCV and NASH.20,[42], [43], [44], [45] Recent translational studies suggested that TH17 cells can be induced by PSC-derived intestinal microbiota and that targeting these TH17 cells improved cholangitis in mice.44 The finding that epigenetic imprinting contributes to the propensity to develop into TH17 cells should spark interest into factors promoting chromatin accessibility, which may even be of microbial origin.
Considering the findings of altered intestinal5,6 and also biliary microbiota4 in patients with PSC, and the increased propensity of T cells to differentiate into TH17 cells upon challenge by pathogens,[44], [45], [46] it is tempting to speculate that microbiota are involved in the activation and differentiation of intrahepatic naive-like CD4+ T cells.44,45,47 Evidence for the differentiation of naive T cells within the liver has already been shown in mice.48 Along this line, we have recently shown that the interplay of biliary epithelial cells and hepatic monocytes, responding to pathogens, promotes a TH17-polarizing microenvironment in the context of PSC.46
Our study has strengths and limitations. We present the first T cell atlas in PSC and therefore in human immune-mediated liver disease. We acquired single-cell sequencing data from 11 livers with PSC, which constitutes an extensive cohort for sequencing studies based on human tissue samples. In addition, we collected peripheral blood of the same patients in parallel to facilitate intra-individual analyses of intrahepatic and circulatory T cells. Taking advantage of this, we provide evidence for CD4+ T cells with both a naive and a tissue-resident phenotype within livers of patients with PSC. Although availability of sufficient numbers of tissue-resident naive-like CD4+ T cells limited the functional in vitro experiments to bona fide naive CD4+ cells from peripheral blood, we show similar developmental propensities for these cells in both compartments. However, to prove that the blood- and liver-derived naive CD4+ T cells are developmentally connected, e.g. blood-derived cells resembling a precursor subset of the tissue-resident cells, further research is needed. As a limitation of our study and due to technical challenges, we acknowledge that explant liver tissue was used for most of the single-cell sequencing studies and future experiments will show whether our findings also hold true in early-stage PSC.
In conclusion, our data indicate that both naive and naive-like CD4+ T cells, in the blood and liver, contribute to the pathogenesis of PSC.
Abbreviations
ALD, alcohol-related liver disease; CITE-Seq, cellular indexing of transcriptomes and epitopes by sequencing; DEG, differentially expressed genes; HDs, healthy donors; LRM, liver resection margin; NASH, non-alcoholic steatohepatitis; PSC, Primary sclerosing cholangitis; scATAC-Seq, single-cell assay for transposase accessible chromatin; scRNA-Seq, single-cell RNA sequencing; scTCR-Seq, single-cell T cell receptor sequencing; TCM, central memory T cell; TCYTOTOXIC, cytotoxic T cell; TEM, effector memory T cell; TFBS, transcription factor binding site; TH1, T helper 1; TH17, T helper 17; TREG, regulatory T cell; tSNE, T-distributed stochastic neighbour embedding.
Financial support
This study was supported by the Deutsche Forschungsgemeinschaft (DFG; CRC841, CRC1192, CRU306, SCHR781/4-1), the Helmut and Hannelore Greve Foundation (CS), the Werner Otto Foundation (JK), the ERC 715271 (NG).
Authors’ contributions
TP, JK, NG and CS designed the study. TP and JK performed the experiments. TP, JK, NG and CS wrote the manuscript. CC, TL and LG performed data analyses. DS supervised experiments and reviewed the manuscript. AF and CK provided the single-cell sequencing platform. MK performed conjugation of the CITE-Seq antibodies. MK and MF helped with establishment of the CITE-Seq protocol. LF, JL and KO provided human biospecimens. AA, LH, AZ, GM, SL and MS organized tissue collection and processed samples for biobanking. ET, MA, AWL and SH supervised the study and reviewed the manuscript.
Data availability statement
The scRNA-Seq data has been deposited in the European Bioinformatics Institute (EBI) ArrayExpress database under accession number E-MTAB-10143 (https://www.ebi.ac.uk/arrayexpress/).
Conflict of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Anna Wöstemeier for discussions and method implementation of the single-cell sequencing. We also thank Alina Borchers, Sabrina Kreß, Jennifer Wigger, Nina Verse, Petra Merkert, Melanie Witt and the FACS Sorting Core Unit of the University Medical Center Hamburg-Eppendorf for excellent technical assistance. We thank Elaine Hussey for language correction of the manuscript. Graphical abstracts were created with BioRender.com.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.03.016.
Contributor Information
Nicola Gagliani, Email: n.gagliani@uke.de.
Christoph Schramm, Email: c.schramm@uke.de.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67(6):1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis K.N., LaRusso N.F. Primary sclerosing cholangitis. N Engl J Med. 2016;375(12):1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boonstra K., Beuers U., Ponsioen C.Y. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Liwinski T., Zenouzi R., John C., Ehlken H., Rühlemann M.C., Bang C. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69(4):665–672. doi: 10.1136/gutjnl-2019-318416. [DOI] [PubMed] [Google Scholar]
- 5.Rühlemann M.C., Heinsen F.-A., Zenouzi R., Lieb W., Franke A., Schramm C. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut. 2017;66(4):753–754. doi: 10.1136/gutjnl-2016-312180. [DOI] [PubMed] [Google Scholar]
- 6.Kummen M., Holm K., Anmarkrud J.A., Nygård S., Vesterhus M., Høivik M.L. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66(4):611–619. doi: 10.1136/gutjnl-2015-310500. [DOI] [PubMed] [Google Scholar]
- 7.Karlsen T.H., Franke A., Melum E., Kaser A., Hov J.R., Balschun T. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138(3):1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Melum E., Franke A., Schramm C., Weismüller T.J., Gotthardt D.N., Offner F.A. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43(1):17–19. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji S.-G., Juran B.D., Mucha S., Folseraas T., Jostins L., Melum E. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49(2):269–273. doi: 10.1038/ng.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aizarani N., Saviano A., Sagar, Mailly L., Durand S., Herman J.S. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572(7768):199–204. doi: 10.1038/s41586-019-1373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran P., Dobie R., Wilson-Kanamori J.R., Dora E.F., Henderson B.E.P., Luu N.T. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575(7783):512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herkel J., Schuchmann M., Tiegs G., Lohse A.W. Immune-mediated liver injury. J Hepatol. 2005;42(6):920–923. doi: 10.1016/j.jhep.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Bogdanos D.P., Gao B., Gershwin M.E. Liver immunology. Compr Physiol. 2013;3(2):567–598. doi: 10.1002/cphy.c120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehal W.Z., Azzaroli F., Crispe I.N. Immunology of the healthy liver: old questions and new insights. Gastroenterology. 2001;120(1):250–260. doi: 10.1053/gast.2001.20947. [DOI] [PubMed] [Google Scholar]
- 15.Schenkel J.M., Masopust D. Tissue-resident memory T cells. Immunity. 2014;41(6):886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar B.V., Connors T.J., Farber D.L. Human T cell development, localization, and function throughout life. Immunity. 2018;48(2):202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eksteen B., Grant A.J., Miles A., Curbishley S.M., Lalor P.F., Hübscher S.G. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200(11):1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eksteen B., Mora J.R., Haughton E.L., Henderson N.C., Lee-Turner L., Villablanca E.J. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137(1):320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant A.J., Goddard S., Ahmed-Choudhury J., Reynolds G., Jackson D.G., Briskin M. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160(4):1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oo Y.H., Banz V., Kavanagh D., Liaskou E., Withers D.R., Humphreys E. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol. 2012;57(5):1044–1051. doi: 10.1016/j.jhep.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liaskou E., Jeffery L.E., Trivedi P.J., Reynolds G.M., Suresh S., Bruns T. Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology. 2014;147(1) doi: 10.1053/j.gastro.2014.04.003. 221-232.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker L.J., Kang Y.-H., Smith M.O., Tharmalingham H., Ramamurthy N., Fleming V.M. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119(2):422–433. doi: 10.1182/blood-2011-05-353789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabo P.A., Levitin H.M., Miron M., Snyder M.E., Senda T., Yuan J. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun. 2019;10(1):4706. doi: 10.1038/s41467-019-12464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durek P., Nordström K., Gasparoni G., Salhab A., Kressler C., Almeida M de. Epigenomic profiling of human CD4+ T cells supports a linear differentiation model and highlights molecular regulators of memory development. Immunity. 2016;45(5):1148–1161. doi: 10.1016/j.immuni.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Yu S., Zhou X., Steinke F.C., Liu C., Chen S.-C., Zagorodna O. The TCF-1 and LEF-1 transcription factors have cooperative and opposing roles in T cell development and malignancy. Immunity. 2012;37(5):813–826. doi: 10.1016/j.immuni.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willinger T., Freeman T., Herbert M., Hasegawa H., McMichael A.J., Callan M.F.C. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol (Baltimore, Md. 1950) 2006;176(3):1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 27.van den Broek T., Borghans J.A.M., van Wijk F. The full spectrum of human naive T cells. Nat Rev Immunol. 2018;18(6):363–373. doi: 10.1038/s41577-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 28.Mahnke Y.D., Brodie T.M., Sallusto F., Roederer M., Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43(11):2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 29.Terada R., Yamamoto K., Hakoda T., Shimada N., Okano N., Baba N. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab Invest J Tech Methods Pathol. 2003;83(5):665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- 30.Kumar B.V., Ma W., Miron M., Granot T., Guyer R.S., Carpenter D.J. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20(12):2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Street K., Risso D., Fletcher R.B., Das D., Ngai J., Yosef N. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC genomics. 2018;19(1):477. doi: 10.1186/s12864-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setty M., Kiseliovas V., Levine J., Gayoso A., Mazutis L., Pe'er D. Characterization of cell fate probabilities in single-cell data with Palantir. Nat Biotechnol. 2019;37(4):451–460. doi: 10.1038/s41587-019-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Backert I., Koralov S.B., Wirtz S., Kitowski V., Billmeier U., Martini E. STAT3 activation in Th17 and Th22 cells controls IL-22-mediated epithelial host defense during infectious colitis. J Immunol (Baltimore, Md. 1950) 2014;193(7):3779–3791. doi: 10.4049/jimmunol.1303076. [DOI] [PubMed] [Google Scholar]
- 34.Chung Y., Yang X., Chang S.H., Ma L., Tian Q., Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16(11):902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 35.McLean C.Y., Bristor D., Hiller M., Clarke S.L., Schaar B.T., Lowe C.B. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28(5):495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentsen M., Goymann P., Schultheis H., Klee K., Petrova A., Wiegandt R. ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat Commun. 2020;11(1):4267. doi: 10.1038/s41467-020-18035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J., Zhang S., Liu Y., He X., Qu M., Xu G. Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell Discov. 2020;6:22. doi: 10.1038/s41421-020-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corridoni D., Antanaviciute A., Gupta T., Fawkner-Corbett D., Aulicino A., Jagielowicz M. Single-cell atlas of colonic CD8+ T cells in ulcerative colitis. Nat Med. 2020;26(9):1480–1490. doi: 10.1038/s41591-020-1003-4. [DOI] [PubMed] [Google Scholar]
- 39.Lo J.C., Chin R.K., Lee Y., Kang H.-S., Wang Y., Weinstock J.V. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest. 2003;112(10):1495–1505. doi: 10.1172/JCI19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langeneckert A.E., Lunemann S., Martrus G., Salzberger W., Hess L.U., Ziegler A.E. CCL21-expression and accumulation of CCR7+ NK cells in livers of patients with primary sclerosing cholangitis. Eur J Immunol. 2019;49(5):758–769. doi: 10.1002/eji.201847965. [DOI] [PubMed] [Google Scholar]
- 41.Grant A.J., Lalor P.F., Hübscher S.G., Briskin M., Adams D.H. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology (Baltimore, Md.) 2001;33(5):1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 42.Lee H.-C., Sung S.-S.J., Krueger P.D., Jo Y.-A., Rosen H.R., Ziegler S.F. Hepatitis C virus promotes T-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatol (Baltimore, Md.) 2013;57(4):1314–1324. doi: 10.1002/hep.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rau M., Schilling A.-K., Meertens J., Hering I., Weiss J., Jurowich C. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/resting regulatory T cell ratio in peripheral blood and in the liver. J Immunol. 2015;196(1):97–105. doi: 10.4049/jimmunol.1501175. [DOI] [PubMed] [Google Scholar]
- 44.Nakamoto N., Sasaki N., Aoki R., Miyamoto K., Suda W., Teratani T. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019;4(3):492–503. doi: 10.1038/s41564-018-0333-1. [DOI] [PubMed] [Google Scholar]
- 45.Katt J., Schwinge D., Schoknecht T., Quaas A., Sobottka I., Burandt E. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology (Baltimore, Md.) 2013;58(3):1084–1093. doi: 10.1002/hep.26447. [DOI] [PubMed] [Google Scholar]
- 46.Kunzmann L.K., Schoknecht T., Poch T., Henze L., Stein S., Kriz M. Monocytes as potential mediators of pathogen-induced Th17 differentiation in patients with primary sclerosing cholangitis (PSC) Hepatology (Baltimore, Md.) 2020 doi: 10.1002/hep.31140. [DOI] [PubMed] [Google Scholar]
- 47.Tilg H., Cani P.D., Mayer E.A. Gut microbiome and liver diseases. Gut. 2016;65(12):2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 48.Klein I., Crispe I.N. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med. 2006;203(2):437–447. doi: 10.1084/jem.20051775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The scRNA-Seq data has been deposited in the European Bioinformatics Institute (EBI) ArrayExpress database under accession number E-MTAB-10143 (https://www.ebi.ac.uk/arrayexpress/).