Abstract
Cardiac sympathetic denervation has been shown to reduce sustained ventricular arrhythmias and implantable cardioverter-defibrillator shocks by inhibiting sympathetic outflow to the heart. We describe the first case to our knowledge of cardiac sympathetic denervation in the left ventricular assist device population. (Level of Difficulty: Advanced.)
Key Words: cardiac assist devices, chronic heart failure, ventricular tachycardia
Abbreviations and Acronyms: AAT, antiarrhythmic therapy; CSD, cardiac sympathetic denervation; ECG, electrocardiogram; HM3, HeartMate 3; ICD, implantable cardioverter-defibrillator; LVAD, left ventricular assist device; NICM, nonischemic cardiomyopathy; VA, ventricular arrhythmias; VT, ventricular tachycardia
Graphical abstract
Introduction
Advanced heart failure results in structural, electrical, and hemodynamic alterations that predispose to ventricular arrhythmias (VAs) (1,2). Risk factors for VAs after left ventricular assist device (LVAD) implantation include electrolyte imbalances, pre-existing scar, ischemia, right ventricular dysfunction, and suction events or ventricular irritation from contact with inflow cannula (2). The single most potent predictor of VAs after LVAD is a history of VAs (2). Cardiac sympathetic denervation (CSD) has been shown to reduce sustained VAs and implantable cardioverter-defibrillator (ICD) shocks by inhibiting sympathetic outflow to the heart (3). We describe the first case to our knowledge of CSD for refractory VAs in a patient with a continuous-flow LVAD.
Learning Objectives
-
•
To understand that the prevalence of VAs in continuous-flow LVADs remains high and requires a multidisciplinary approach to treatment.
-
•
To introduce cardiac sympathetic denervation for refractory VAs in patients with a continuous-flow LVAD.
History of presentation
A 46-year-old woman with a history of nonischemic cardiomyopathy (NICM) with an LVAD presented with palpitations and an ICD shock. The patient’s mean arterial pressure was 68 mm Hg, heart rate was 60 beats per minute, and respiratory rate was 20 breaths/min. She was afebrile. The physical examination results were remarkable for an audible LVAD hum and no palpable pulse. The electrocardiogram (ECG) revealed sequential atrial and ventricular pacing. Laboratory analysis showed a hemoglobin of 13.8 g/dl, potassium of 4.5 mEq/l, magnesium of 1.9 mg/dl, creatinine of 1.3 mg/dl, thyroid-stimulating hormone of 11.83 IU/ml, and free thyroxine Ft4 of 1.6 ng/dl. Over the past 8 months before this current presentation, the patient had experienced at least 5 episodes of VAs requiring anti-tachycardia pacing or cardioversion/defibrillation. The first episode of VA occurred 1.5 years after the LVAD implantation. She was already receiving antiarrhythmic therapy (AAT) with amiodarone 400 mg daily and mexiletine 150 mg twice daily.
Medical History
Peripartum cardiomyopathy status after HeartMate 3 (HM3) LVAD implantation and tricuspid valve annuloplasty 2 years before presentation.
Differential Diagnosis
Ventricular tachycardia was secondary to underlying structural heart disease.
Investigations
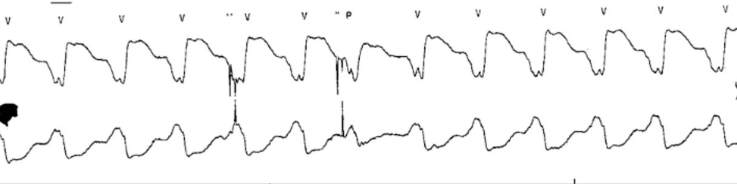
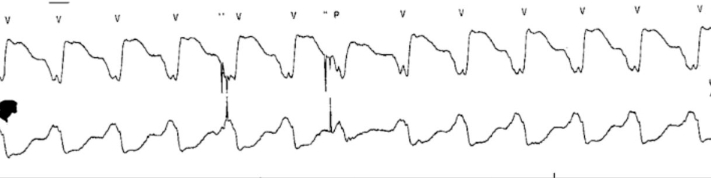
Laboratory markers revealed preserved end-organ function. There were no low-flow or suction events on the LVAD interrogation. Echocardiography revealed stable right ventricle function, biventricular chamber size, and LVAD cannular position. The patient was evaluated by the electrophysiology service and deemed not to be a candidate for ventricular tachycardia (VT) ablation due to technical difficulties with epicardial access in the presence of an LVAD and overall low likelihood of success given nonischemic substrate. Of note, the patient was also listed for cardiac transplantation as United Network for Organ Sharing Status 4 and was highly sensitized, with a calculated Panel of Reactive Antibodies of 63% for HLA class I antigens and of 100% HLA class II antigens. On hospital day 3, the patient experienced palpitations, and telemetry (Figure 1) showed slow VT, despite dual AAT.
Figure 1.
Telemetry
The telemetry tracing shows an extremely wide QRS (220 ms) concerning ventricular tachycardia.
Management
Given recurrent VA refractory to multiple AAT, we considered requesting a status upgrade for urgent transplantation; however, her highly sensitized status presented considerable risk without time to implement desensitization therapies. After a multidisciplinary discussion about available treatment options, we decided to proceed with CSD, and a thoracic surgeon performed video-assisted thoracoscopy with bilateral stellectomy. After the procedure, the patient remained symptom-free/arrhythmia-free and was discharged 5 days later.
Discussion
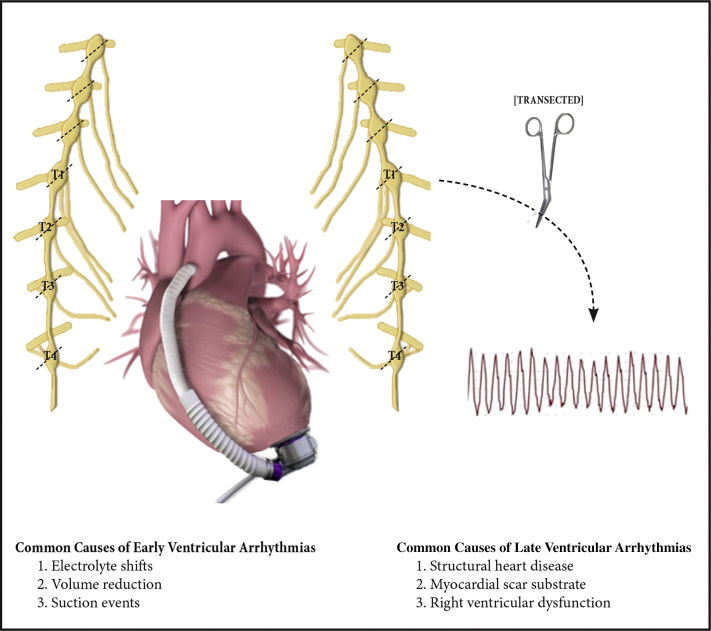
Early VAs are defined as occurring within the first 30 days after LVAD implantation and usually occur in the setting of post-operative inotropes, electrolyte shifts, aggressive left ventricular unloading, volume reduction, and suction events, or variability in LVAD parameters (4). A multicenter study of 652 patients with LVADs found that 25% of all patients experienced early VAs, most likely in the first week (5). Furthermore, early VAs were significantly associated with premature post-operative mortality, which is augmented 7-fold with an electrical storm.
Late VAs, defined as occurring after the first month of LVAD implant, result from underlying structural heart disease and myocardial scar substrate, new apical scarring in response to suture lines, or right ventricle dysfunction (4). The mortality impact of VA on the LVAD population is variable because the risk of sudden cardiac death is decreased (6). A 15-year retrospective analysis of 517 patients with LVADs found an increase in mortality with early VAs, which correlated with right ventricular failure (7). Late VAs were not associated with an increase in mortality.
The need for an ICD after LVAD implantation continuous to remains debatable. Current American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines provide a Class IIa recommendation for ICD implantation in patients with LVAD with a sustained VA (4). Concerns for infection, electrical interference, and inappropriate shocks have limited the role of ICDs. Current studies do not show a survival benefit from ICD therapy in patients with LVADs (8). The most recent literature is only in the Heartmate II or HeartWare Ventricular Assist Device population, and data on the Heartmate 3 population is scarce.
Catheter ablation is usually considered for recurrent VA refractory to multiple AAT and ICD therapies. Potential challenges with ablation in the LVAD population include technical difficulty with epicardial access and concern for catheter entrapment. ECGs also may be unreliable in consistently predicting VA location, possibly due to anatomic distortion from LVAD placement. Procedural success ranged from 77% to 86% (4). Freedom from recurrent VAs after ablation correlated with better 1-year survival (9).
CSD has been shown to reduce the incidence of sustained VAs and recurrent ICD shocks in patients with VAs refractory to medical or catheter ablation therapies (3). In CSD, the lower one half of the stellate ganglion and the thoracic ganglia at T2 to T4 are transected, removing post-ganglionic efferent activity that usually acts on the heart in response to blood pressure and heart rate stimuli (Figure 2). The most extensive retrospective review of specialized centers with experience in performing CSD looked at 121 patients with structural heart disease who underwent CSD for recurrent VT or VT storm (3). Seventy-one percent of patients had a NICM, with a mean left ventricular ejection fraction of 30%, and underwent either left or bilateral CSD using video-assisted thoracoscopic surgery. Fifty-eight percent of the patients were free of ICD shocks or sustained VT at 1 year, and about 33% of patients no longer required antiarrhythmic medications. The authors concluded patients with NICM are more challenging for catheter ablation and would be the target population for CSD. A recent meta-analysis included 311 patients who underwent CSD for refractory VT or electrical storm and found a 60% rate of freedom from VT at a mean follow-up of 15 months (10).
Figure 2.
Mechanism of Bilateral Cardiac Sympathetic Denervation in Reduction of Ventricular Arrhythmias
The lower one half of the stellate ganglion and the thoracic ganglia at T1 to T4 was transected bilaterally, interrupting the sympathetic input to the heart and reducing ventricular tachycardia recurrence.
In our case of late VA post-LVAD, which was refractory to AAT and not amendable to catheter ablation, CSD led to at least a temporary resolution of the VAs. We have found no prior reports of CSD therapy being used in patients with LVADs. We should note first-line CSD therapy for VAs post-LVAD is not suitable with current guidelines. This report shows that CSD can be safely done in patients with LVADs with VAs and this could reduce VA burden and the need for ICD therapies. Catheter ablation in HM3 LVADs has been less studied, with some reports of HM3 causing high-frequency noise on the surface ECG, making magnet-based mapping problematic. Therefore, CSD could be considered where catheter ablation is not feasible. Also, a decrease in VA burden might allow for amiodarone discontinuation, which is important because amiodarone use in the LVAD population has been demonstrated to increase primary graft failure risk.
Follow-Up
On outpatient follow-up 8 months after the procedure, the patient continued to remain free of VAs.
Conclusions
This is the first case to our knowledge of CSD in the LVAD population. CSD can be considered in patients with LVADs who are unresponsive to pharmacological, catheter ablation, or ICD therapies for VAs.
Funding Support and Author Disclosures
Dr. Goldstein has served as a consultant for Abbott. Dr. Jorde has served as a consultant for Abbott. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. The authors D.G. and U.J. are consultants for Abbott. For more information, visit the Author Center.
References
- 1.Santangeli P., Rame J.E., Birati E., Marchlinski F. Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol. 2017;14:1842–1860. doi: 10.1016/j.jacc.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Garan A.R., Yuzefpolskaya M., Colombo P.C. Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol. 2013;25:2542–2550. doi: 10.1016/j.jacc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Vaseghi M., Barwad P., Malavassi Corrales F.J. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol. 2017;25:3070–3080. doi: 10.1016/j.jacc.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopinathannair R., Cornwell W.K., Dukes J.W. Device therapy and arrhythmia management in left ventricular assist device recipients. Circulation. 2019;20:e967–e989. doi: 10.1161/CIR.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 5.Galand V., Flécher E., Auffret V. Early ventricular arrhythmias after LVAD implantation is the strongest predictor of 30-day post-operative mortality. J Am Coll Cardiol EP. 2019;8:944–954. doi: 10.1016/j.jacep.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Galand V., Flécher E., Auffret V. Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. J Am Coll Cardiol EP. 2018;9:1166–1175. doi: 10.1016/j.jacep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Greet B.D., Pujara D., Burkland D. Incidence, predictors, and significance of ventricular arrhythmias in patients with continuous-flow left ventricular assist devices: a 15-year institutional experience. J Am Coll Cardiol EP. 2018;2:257–264. doi: 10.1016/j.jacep.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Enriquez A.D., Calenda B., Miller M.A. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol. 2013;4:668–674. doi: 10.1161/CIRCEP.113.000457. [DOI] [PubMed] [Google Scholar]
- 9.Moss J.D., Flatley E.E., Beaser A.D. Characterization of ventricular tachycardia after left ventricular assist device implantation as destination therapy: a single-center ablation experience. J Am Coll Cardiol EP. 2017;12:1412–1424. doi: 10.1016/j.jacep.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Murtaza G., Sharma S.P., Akella K. Role of cardiac sympathetic denervation in ventricular tachycardia: a meta-analysis. PACE. 2020;43:828–837. doi: 10.1111/pace.13968. [DOI] [PubMed] [Google Scholar]