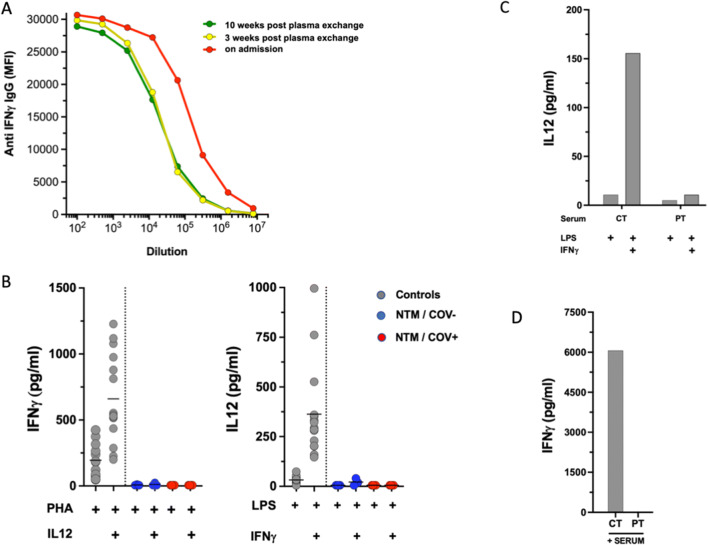
Fig. 1.
A Detection and titration of anti-IFNγ antibody. Patient sera were diluted in seven 1/5 steps starting at 1/100. Anti-IFNγ IgG was determined by particle-based flow cytometry on a Luminex analyzer and shown as mean fluorescence intensities (MFI). B Induced IFNγ and IL12 levels after whole blood activation. Cytokines were measured in whole blood after 24-h stimulation using 10 μg/mL PHA or 1 μg/mL LPS alone or in combination with 20 μg/mL IL12 or 20,000 IU/mL IFNγ, respectively. To account for lymphopenia, data were corrected for lymphocyte counts (in response to PHA) or monocyte counts (in response to LPS). Controls are shown as gray circles (N = 15), patients with acquired (a)IFNγ deficiency (N3) are shown as blue circles (NTM/COV−). For the NTM/COV+ patient, data from four experiments are shown as red circles. Cytokine levels are shown as pg/mL. C In vitro inhibition of IFNγ using patient serum. Healthy control PBMC were activated with LPS or LPS + IFNγ in the presence of 20% control (Ct) or patient (NTM/COV+) serum and IL12 levels were measured in the supernatant after 24-h incubation. D IFNγ recovery in the presence of autologous patient (NTM/COV+) serum: 20% control or patient serum was added to RPMI-containing recombinant IFNγ and IFNγ levels were determined by Luminex. No IFNγ could be recovered from the well containing patient serum showing complete antigenic neutralization by the patient’s autoantibody