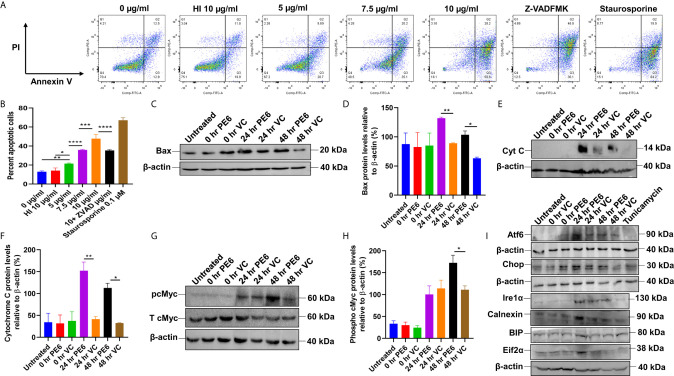
Figure 6.
PE6 induced apoptosis activation by inducing pro-apoptotic factors and efficiently activating the ER stress-mediated UPR pathway. (A) Flow cytometric analysis of early apoptotic cells of PE6 treated macrophage cells. Untreated and HI PE6 treated cells were used as negative controls. In contrast, staurosporine and ZVAD-FMK treated cells served as positive and negative controls for caspase-dependent induction and apoptosis repression. Briefly, RAW264.7 cells were seeded in a 6-well tissue culture plate, after 2 h of adherence at 37°C, the cells were treated with PE6 (5, 7.5, and 10 µ/ml), HI PE6 (10 µg/ml), 0.1 µM staurosporine, and 20 µM pan-caspase inhibitor Z-VAD-FMK. After completing the treatment, cells were harvested and processed as instructed by the manufacturer (BD Biosciences, San Jose, USA). Sample’s reading was captured using the BD FACSVerse machine. Images were analyzed using FlowJo software. (B) Graphical representation of apoptosis induction by treatment of various concentrations of PE6. (C) Western blot analysis of recombinant M. smegmatis infected macrophage cells after 24 and 48 h of infection. Western blot was performed using an anti-Bax antibody. (D) Densitometric analysis of Bax protein levels normalized to β-actin. (E) Western blot showing the levels of Cytochrome C protein in macrophage cells infected with recombinant M. smegmatis. (F) Densitometric quantitation of Cytochrome C protein levels relative to β-actin represented as a bar graph. (G) Western blot analysis of pcMYC levels in recombinant M. smegmatis infected macrophage cells. (H) Densitometric quantitation of pcMYC levels relative to β-actin and represented as a bar graph. Untreated and vector alone transformed cells were used as negative controls. Protein levels were represented as [%] to β-actin and total cMYC. Data were analyzed by one-way ANOVA with Tukey’s multiple comparison post-test. Data are representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. controls. (I) Western blot analysis showing the levels of UPR markers in recombinant M. smegmatis infected macrophage cells. RAW264.7 macrophage cells were infected with recombinant M. smegmatis at MOI of 1:10 for 24 and 48 h. The cell lysate was prepared, run in SDS-PAGE, transferred onto the PVDF membrane, and Western blotted using anti-Atf6, anti-Chop, Ire1α, Calnexin, BIP, and Eif2α antibodies. Untreated and vector alone transformed cells were used as controls. β-actin was used as a loading control.