Abstract
Schizophrenia is a severe psychiatric disorder characterized by emotional, behavioral and cognitive disturbances, and the treatment of schizophrenia is often complicated by noncompliance and pharmacoresistance. The search for the pathophysiological mechanisms underlying schizophrenia has resulted in the proposal of several hypotheses to explain the impacts of environmental, genetic, neurodevelopmental, immune and inflammatory factors on disease onset and progression. This review discusses the newest insights into the pathophysiology of and risk factors for schizophrenia and notes novel approaches in antipsychotic treatment and potential diagnostic and theranostic biomarkers. The current hypotheses focusing on neuromediators (dopamine, glutamate, and serotonin), neuroinflammation, the cannabinoid hypothesis, the gut-brain axis model, and oxidative stress are summarized. Key genetic features, including small nucleotide polymorphisms, copy number variations, microdeletions, mutations and epigenetic changes, are highlighted. Current pharmacotherapy of schizophrenia relies mostly on dopaminergic and serotonergic antagonists/partial agonists, but new findings in the pathophysiology of schizophrenia have allowed the expansion of novel approaches in pharmacotherapy and the establishment of more reliable biomarkers. Substances with promising results in preclinical and clinical studies include lumateperone, pimavanserin, xanomeline, roluperidone, agonists of trace amine-associated receptor 1, inhibitors of glycine transporters, AMPA allosteric modulators, mGLUR2-3 agonists, D-amino acid oxidase inhibitors and cannabidiol. The use of anti-inflammatory agents as an add-on therapy is mentioned.
Keywords: Schizophrenia, Immune system, Inflammation, Genetics, Novel antipsychotics, Add-on therapy
Core Tip: This review discusses the newest insights in the pathophysiology and risk factors for schizophrenia and points out the novel approaches of antipsychotic treatment, potential diagnostic and theranostic biomarkers. The hypotheses focusing on neuromediators (dopamine, glutamate, serotonin), neuroinflammation, cannabinoid hypothesis, gut brain axis model, and other currently discussed hypotheses are summarized. Key genetic features and new findings in the pathophysiology of schizophrenia support the expansion of novel approaches in pharmacotherapy and development of non-dopaminergic antipsychotics.
INTRODUCTION
Schizophrenia is a serious mental disorder with a lifelong prevalence of approximately 1% and a peak age of onset of 23-34 years in women and the early twenties in men. It is a very complex syndrome that involves widespread brain multi-dysconnectivity. It is characterized by cognitive, behavioral and emotional dysfunctions. To fulfil the diagnostic criteria for schizophrenia, patients must exhibit two or more negative, disorganized or positive symptoms that persist for a minimum of six months, and at least one symptom must be disorganized speech or a positive symptom[1]. Positive symptoms include hallucinations and delusions; negative symptoms are characterized by deficits in normal behavior, including asociality, alogia, anhedonia, blunted affect, and avolition[2]. There is a wide range of treatment possibilities; however, the effectiveness and/or adverse effects of antipsychotics with different pharmacological profiles vary. Successful treatment of schizophrenia is complicated by noncompliance and pharmacoresistance. The prevalence of pharmacoresistant schizophrenia is estimated to range from 12.9% to 48%[3]. It has been estimated that approximately 20% of patients with schizophrenia receive combination treatment and/or antipsychotic polypharmacy[4]. Augmentation strategies used in clinical practice include the addition of another antipsychotic, concurrent administration of benzodiazepines or mood stabilizers, repetitive transcranial magnetic stimulation or electroconvulsive therapy.
The pathophysiological mechanism of the onset and progression of schizophrenia, the diagnostic neuropathology, and sensitive and specific biomarkers have not yet been identified. Several different hypotheses have been proposed to explain the neuropathology of schizophrenia that focus on environmental, genetic, neurodevelopmental, and neurochemical effects. Research and development in imaging methods and in preclinical studies have led to the improvement of these theories. Positron emission tomography (PET) and single photon emission computer tomography enable in vivo quantification of dopaminergic functions in the brain and dopamine synthesis, release, and availability in postsynaptic dopaminergic neurons and transporters.
The targeting of existing and new drugs is based primarily on the dopamine and glutamate hypotheses of schizophrenia. All current antipsychotics modulate the function of the dopamine D2 receptor. A nonlinear relationship between D2 receptor occupancy, clinical response, and adverse effects of current antipsychotics was found. A small response to antipsychotic treatment appears at 50% dopamine receptor occupancy; as receptor occupancy increases, the response increases as well as the risk of extrapyramidal adverse effects[5]. These findings were proven in a double-blind study in patients with first episode schizophrenia; 65% occupancy of D2 receptors was the borderline between responders and nonresponders[6]. Recently, research has focused on the prodromal phase of schizophrenia. Dopamine synthesis increases during the acute phase of the disease. Stress and other risk factors affect the dopamine systems, leading to their dysregulation and consequently to the development of psychotic disorder[7].
Excitatory glutamate neurotransmission occurs though ionotropic and metabotropic glutamate receptors. The glutamate hypothesis of schizophrenia is based on the dysfunction of the N-methyl-D-aspartate (NMDA) receptor. Currently, the effects of ketamine on brain function in healthy volunteers are being examined; studies are focused on glutamate concentrations in the brains of patients with prodromal symptoms during the first episode and other episodes of schizophrenia. Dysfunction of both NMDA receptors and presynaptic synthesis of dopamine has been implicated in the clinical symptoms of schizophrenia. Relationships between presynaptic dopamine dysfunction and positive symptoms and between glutamate dysfunction and negative and cognitive symptoms are expected[7].
To improve the diagnosis of schizophrenia, predict the therapeutic response to antipsychotics, develop new drugs, and personalize treatment, it is necessary to identify new specific and sensitive biomarkers of the disease[8]. Blood-based biomarkers are regarded as a feasible option because the dysregulation of gene expression, epigenetic patterns, protein quantities, and metabolic and inflammatory molecules in peripheral blood have been shown to have distinct patterns in patients with schizophrenia[8]. The aim of this review is to provide the newest insights into the pathophysiology and risk factors of schizophrenia and novel approaches to antipsychotic treatment.
GENETICS AND SCHIZOPHRENIA
Schizophrenia is closely linked to genetic factors, including small nucleotide polymorphisms (SNPs), copy number variations and changes in gene expression. Combinations of different pathogenic mechanisms, including aberrant DNA methylation, altered histone code, dysregulated long noncoding RNA (lncRNA)-dependent tethering of epigenetic complexes to DNA, aberrant polyadenylation of pre-mRNAs, and mis-splicing, have been reported to play a role in schizophrenia development[9]. The hereditary burden of schizophrenia is estimated to be approximately 80%. Genome-wide association studies (GWAS) have identified more than 100 loci, many of which contain multiple genes that are significantly associated with schizophrenia. The assessment of polygenic scores allows us to determine the risk of schizophrenia based on the number of risk alleles weighted by the odds ratio of each allele.
DNA methylation, an epigenetic process that produces 5-methylcytosine, is mediated by DNA methyltransferases and has a key role in several processes, such as imprinting, inactivation of the X-chromosome, silencing of transposons or regulation of genomic stability and chromatin structure. Schizophrenia is linked to pathophysiological DNA methylation of several genes, including those encoding reelin, catechol-O-methyltransferase (COMT), monoamine oxidase A, serotonin receptor 2A, the transcription factor SOX-10, and others. Unfortunately, no schizophrenia-specific “methylation panel” has been proposed, and it has not yet been clarified whether these changes represent causes or consequences of schizophrenia development[9].
Approximately 70%-80% of the genome is transcribed into noncoding transcripts, and the majority of schizophrenia-associated risk variants have been found in noncoding regions. LncRNAs can interact with DNA, RNA, and proteins, influencing transcription and posttranscriptional processes such as splicing, polyadenylation and/or regulation of transcript stability. MicroRNAs (miRNAs) are small noncoding RNAs that regulate more than 50% of protein-coding genes by acting as promoter or enhancer elements; miRNAs might participate in histone, DNA, or chromatin methylation and modification. Both lncRNAs and miRNAs can be affected by different genetic variants, especially SNPs, which could increase the risk of schizophrenia onset[9,10].
Microdeletions in chromosomal region 22q11.2 are one of the well-established genetic risk factors for schizophrenia and increase the risk of schizophrenia development to 30%-40%[11,12]. COMT is a major dopamine catabolic enzyme, and its gene is located in this microdeletion region. In addition, a functional COMT polymorphism [valine/methionine (VAL/MET) substitution at codon 108] causes differences in its catabolic activity, dopamine baselines and stress-induced cortical dopamine release[13]. The MET version of the allele is not as stable as the VAL version, causing decreased COMT activity and an increase in dopamine levels, especially in the prefrontal cortex[14].
The major histocompatibility complex (MHC) locus located on chromosome 6, which contains genes encoding proteins essential for adaptive immunity, has one of the strongest links to schizophrenia. Specifically, there was increased expression of complement component 4A (C4A). Sex differences in the C4 gene could explain the higher male susceptibility to schizophrenia. Schizophrenia patients with higher C4 Levels were characterized as low responders or nonresponders to antipsychotic medication. The expression of the genes encoding CSMD1 and CSMD2, which are important regulators of C4, has been found to be decreased in schizophrenia and connected with reduced cognition and executive function[15,16]. Other immune receptors, including toll-like receptors (TLRs), which take part in microbe-derived molecular signaling, early brain development, synaptic plasticity, and neurogenesis, have been identified as schizophrenia susceptibility genes by GWAS. Both TLR2 and TLR4 were altered in the blood and brain tissue of schizophrenic patients[15].
The genes encoding for neuregulin 1 and neuregulin 3 are candidate schizophrenia genes and produce several possible proteins that influence neuronal differentiation and migration. The role of neuregulin 1 in schizophrenia is not well known, but increased neuregulin 1 signaling led to NMDA receptor hypofunction (in accordance with the glutamate hypofunction hypothesis of schizophrenia). There is no evidence of hyperexpression of neuregulin 1 itself; however, the possibility of mutations causing the production of proteins with enhanced function is still present[14]. Neuregulin 3 is a ligand for receptor tyrosine-protein kinase erbB-4 (ErbB4), and different genetic variants of the neuregulin 3 gene, especially the rs10748842 allele, are connected with higher schizophrenia risk and cognitive impairment[17]. Mutant mice with ErbB4 deletion from fast-spiking interneurons exhibited increased cortical excitability and oscillatory activity and desynchronized neurons in the cortical region, probably caused by the disruption of the proper function of inhibitory GABA circuits in interneurons. These functional changes manifested in increased locomotion, impaired social and emotional behavior and cognitive dysfunction, which are common symptoms of schizophrenia[18,19].
The gene encoding dystrobrevin-binding protein 1 (also referred to as dysbindin or DTNBP1) has been identified as a gene associated with schizophrenia; however, no specific protein coding mutations increasing the risk of schizophrenia have been identified. Decreased dysbindin expression has been found in the brains of schizophrenia patients, and dysbindin risk haplotypes have been associated with increased negative symptomatology in schizophrenia[14].
The gene most closely linked to schizophrenia is probably the gene encoding the protein disrupted in schizophrenia 1 (DISC1), which has been associated with schizophrenia mainly due to a mutation causing a translocation between exons 8 and 9. The molecular mechanism of this mutation is not known, but the shortened mutant DISC1 protein is incapable of dimerization, and it may interact with other proteins. DISC1 expression is especially high during neurodevelopment in the late fetal and early postnatal phases, during which it participates in hippocampal development; however, DISC1 expression continues into adulthood. In schizophrenia pathophysiology, not only DISC1 itself but also its binding and interaction partners, such as microtubule-associated protein 1A, glycogen synthase kinase 3β, phosphodiesterase 4 and fasciculation and elongation protein zeta-1, might play a crucial role[14,20-22].
The synaptosomal-associated protein SNAP25 is involved in synaptic vesicle docking and fusion during neurotransmitter release. The promoter variant rs6039769 with the C risk allele caused an increase in SNAP25 expression, probably causing a larger amygdala and greater functional connectivity between the amygdala and ventromedial prefrontal cortex in male schizophrenic patients. This modulation in the plasticity of the prefrontal cortex-limbic connection caused higher schizophrenia risk[23].
The gene encoding transcription factor 4 (TCF4) is another GWAS-confirmed gene associated with schizophrenia. It encodes class I basic helix-loop-helix transcription factors and plays a role in neurodevelopment. Altered expression of TCF4 in the forebrain of a transgenic mouse caused altered cognition and long-term depression increased the density of immature spines[24]. Many other genes have been associated with schizophrenia diagnosis and have been reported in the literature[25-27]; description of all schizophrenia-linked genes is beyond the scope of this review.
TRIGGERS AND RISK FACTORS
Environmental model of schizophrenia
The onset and severity of schizophrenia are always modulated by an interplay between genetic and environmental risk factors[28]. Many epidemiological studies have investigated putative environmental risk factors for schizophrenia and peripheral biomarkers of the disease[29,30]. According to an umbrella review of meta-analyses on risk factors and peripheral biomarkers for schizophrenia[31], history of obstetric complications, exposure to stressful events in adulthood or to childhood adversity, cannabis use, and serum folate level showed robust evidence of association with schizophrenia.
The prenatal and perinatal periods are characterized by great neural vulnerability to environmental insults. A recent systematic review and meta-analysis of 152 studies revealed numerous prenatal and perinatal risk factors, calculated with odds ratios (ORs), that were statistically linked to schizophrenia onset[32]. The biggest risk factors for schizophrenia onset are any familial psychopathology, especially maternal psychosis (OR: 7.61). Maternal infections (herpes simplex 2, OR: 1.35; unspecified infections, OR: 1.27), a suboptimal number of antenatal care visits (OR: 1.83), or maternal stress (OR: 2.4) can lead to a higher prevalence of obstetric events (OR: 1.52), which are the longest-studied and best replicated environmental risk factors for schizophrenia. Significantly relevant obstetric events include maternal hypertension (OR: 1.4), hypoxia (OR: 1.63), premature rupture of membranes (OR: 2.29) and polyhydramniosis (OR: 3.05). There is experimental and clinical evidence showing significant risks of prenatal infection and inflammation for the later development of schizophrenia. According to the viral model of schizophrenia, prenatal viral and bacterial infections and inflammation play an important role in the development of schizophrenia[33].
Nutritional deficits or famine in pregnancy (OR: 1.4) or more than two pregnancies (OR: 1.3) can be associated with reduced allocation or lower socioeconomic status. Another risk factor is congenital malformations (OR: 2.35)[32]. The most relevant postnatal environmental risk factors are childhood trauma (OR: 2.87), urban living (OR: 2.19), migration (2.10) and cannabis use (OR: 5.17), and these stress factors lead to the sensitization of the subcortical dopamine system[11].
Many genes relevant to schizophrenia, especially immune genes, can be altered by air pollution. Children with greater exposure to traffic-related air pollution had increased levels of proinflammatory cytokines. It is not yet clear whether air pollution itself causes brain changes or inflammatory changes caused by air pollution contribute to the pathology of schizophrenia[15].
A study of the roles of both genetic and environmental influences on the development of schizophrenia is necessary to explain the fact that in approximately 40%-55% of cases, monozygotic twins do not share a diagnosis of schizophrenia[34]. How genetic and environmental factors interact and the related neurobiological mechanisms that induce schizophrenia are not yet known.
Stress and schizophrenia
The vulnerability-stress model of schizophrenia proposes that when stress exceeds the vulnerability threshold, an individual is likely to develop a psychotic episode[35]. Stressful life events or psychological stress, especially in key periods of neurodevelopment, increase the risk of schizophrenia. These events include physical or mental abuse, lower socioeconomic status, urban environment, and neglect. The molecular mechanisms connecting these stressful situations with schizophrenia remain unclear. It was proven that patients with schizophrenia have altered cortisol function, and its release is linked to the inflammatory response rather than the anti-inflammatory response. Observation of HPA axis activation and cortisol release as a result of stress events in individuals with schizophrenia has produced inconsistent results; however, HPA axis dysfunction has been observed[15].
Neurons are extremely sensitive to redox imbalance during neurodevelopment and differentiation, mostly because of their high lipid content and metabolic rate. Increased reactive oxygen species (ROS) production and/or lowered antioxidant system capacity are considered risk factors for schizophrenia development. Increased protein and lipid oxidation and lowered levels of vitamin C and E, catalase, glutathione peroxidase and superoxide dismutase have been detected in schizophrenia patients. A study revealed that participants with low vitamin D3 Levels in the first year of life were at two times higher risk of schizophrenia. Glutamate-cysteine ligase is the rate-limiting biosynthetic enzyme of glutathione. One allelic variant of the GCLC gene is linked to the decreased activity of glutamate-cysteine ligase and schizophrenia. NMDA receptors are regulated by the redox state, and glutathione deficiency induces NMDA receptor hypofunction, which leads to cortical oxidative stress and glutathione decrease[36,37].
Neurodevelopmental model
The neurodevelopmental model postulates that an increased risk of schizophrenia development is the result of abnormal brain neurodevelopment caused by genetic and environmental factors years before the onset of the disease[38]. The hypothesis is based on clinical, epidemiological, brain imaging, and genetic studies[39,40]. Schizophrenia is supposed to be a developmental disorder of the brain, and changes in brain neuroplasticity are involved. The disconnection hypothesis[41] presumes the involvement of abnormal synaptic connections in the pathophysiology of schizophrenia. Impaired synaptic plasticity and synaptic efficacy, mainly in areas of the brain responsible for learning, memory, and emotion, participate in schizophrenia pathophysiology. Modulation of ascending neurotransmitter systems and consolidation of synaptic connections during learning are implicated in schizophrenia neuropsychology, especially in impaired adaptive behavior and disintegrative aspects[42].
The unitary hypothesis of schizophrenia includes different types of pathophysiological models[43]; according to the hypothesis, early brain insults can lead to dysplasia of selective neural circuits, which is responsible for premorbid cognitive and psychosocial dysfunction in patients with schizophrenia. The onset of psychosis in adolescence may be associated with the excessive elimination of synapses with subsequent dopaminergic over activity. Decreased glutamatergic neurotransmission can predispose the brain to these processes. After the onset of the disease, these neurochemical changes can lead to further neurodegenerative processes. Brain plasticity includes both synaptic and nonsynaptic plasticity. The dysplastic model of schizophrenia suggests that impaired neuroplasticity during brain development may underlie cognitive and deficit symptoms and may lead to reorganization in other neuronal circuits, which may lead to affective and psychotic symptoms[44].
The multiple hit theory of schizophrenia[45] presumes that schizophrenia can be conceptualized as a process involving multiple vulnerability factors across numerous neurodevelopmental windows in which some hits are applied prenatally, in childhood, in adolescence, and in adulthood. Thus, the development of schizophrenia is driven by the interactions between genetic vulnerability and environmental influences (including prenatal vitamin D, nutrition, childhood trauma, viral infections, IQ, smoking, cannabis use, and social defeat), which are cumulative and interact with each other. The neurodevelopmental phase involves changes in synaptogenesis, synaptic enhancement, and myelination, leading to excessive elimination of synapses and loss of neuroplasticity.
An extension of the neurodevelopmental model[46] proposes that the abnormal formation and maturation of connectomes (an extensive network of interconnected neurons) is central to the etiology of the disease. That is, abnormal anatomical architecture and functional organization of the connectome may be a final common pathway leading to the manifestation of schizophrenia symptoms. To further refine the developmental hypothesis of schizophrenia, progress in our understanding of brain connectivity during development and dysconnectivity resulting from genetic and environmental factors is necessary.
Oxidative stress and apoptosis
Disconnection of the prefrontal cortex in schizophrenic patients is associated with abnormalities in white matter, oligodendrocytes, and myelin. Myelin is produced by mature oligodendrocytes, and oligodendrocyte precursor cells are extremely sensitive to oxidative stress. A redox-induced prefrontal oligodendrocyte precursor cell-dysfunctioning hypothesis of cognitive symptomatology in schizophrenia has been proposed[47]. According to this hypothesis, the combination of environmental factors and genetic predisposition causes oxidative stress due to the excessive generation of ROS and reactive nitrogen species in oligodendrocyte precursor cells. Oxidative stress can lead to the downregulation of myelin-related genes in oligodendrocytes, decreased expression of myelin basic protein, and a reduced number of oligodendrocytes in the rat brain. During adolescence, a high concentration of ROS impairs the proliferation and differentiation of oligodendrocytes and their precursors. This leads to their dysfunction and hypomyelination and consequently to the disruption of connectivity in the prefrontal cortex. The resulting cognitive symptoms coincide with the onset of schizophrenia.
Additionally, oxidative stress induces dysregulation of the immune system and favors a proinflammatory response. Inflammation and disruption of immunity are other factors contributing to the pathogenesis of schizophrenia, as described in the following sections.
Mitochondria play a major role in cellular bioenergetics, oxidative stress, and apoptosis. According to the mitochondrial hypothesis of schizophrenia, mitochondrial dysfunction leads to distorted neuronal activity and plasticity, causing imbalanced brain circuitry and finally abnormal behavior[48]. Massive loss of white matter oligodendrocytes is a hallmark of schizophrenia. Therefore, it has been hypothesized that mitophagy is increased in oligodendrocytes in schizophrenia, which contributes to disease-related white matter neuropathology.
The intrinsic pathway of apoptosis is activated by intracellular signals generated during cellular stress and is triggered by the release of proapoptotic factors from mitochondria. Thus, consistent with the mitochondrial hypothesis, the apoptotic hypothesis postulates that apoptosis contributes to the pathophysiology of schizophrenia. The data indicate a dysregulation of apoptosis in several cortical areas in schizophrenia. The potential involvement of nonlethal localized apoptosis in the early stages of the disease is presumed[49].
NEUROCHEMICAL HYPOTHESES
Dopamine hypotheses
According to the classic (receptor) dopamine hypothesis of schizophrenia, psychotic symptoms are related to dopaminergic hyperactivity in the brain. Hyperactivity of dopaminergic systems during schizophrenia is the result of increased sensitivity and density of dopamine 2 (D2) receptors. This increased activity can be localized in specific brain regions[50,51]. The dopamine hypothesis does not assume that dopamine hyperactivity fully explains schizophrenia. Over activation of D2 receptors appears to be only one effect of the overall dysregulation of chemical synapses in this disease.
The modified dopamine hypothesis assumes that schizophrenia is characterized by abnormally low prefrontal dopamine activity (causing negative symptoms) that leads to excessive dopamine activity in mesolimbic dopamine neurons (causing positive symptoms). Thus, this hypothesis presumes the co-occurrence of high and low dopamine activity in different neuronal circuits, which could explain the concurrent presence of positive and negative symptoms[52].
The unifying dopamine hypothesis of schizophrenia, called "the final common pathway", proposes that multiple environmental, genetic, and other risk factors (such as stress, drugs, or frontotemporal dysfunction) interact and result in striatal dopamine dysregulation, which alters signal transmission and leads to psychosis[53]. This hypothesis combines dopamine dysfunction with other risk factors, including pregnancy and obstetric complications, stress and trauma, drug abuse, genetic predisposition and environment–gene interactions, with both increased presynaptic striatal dopaminergic function and other brain functions that underlie negative and cognitive symptoms.
A model has been presented of how genes and environmental factors may sensitize the dopamine system so that it is vulnerable to acute stress, leading to progressive dysregulation and the onset of psychosis[13]. The main steps of this model are as follows: genetic risk factors lead to impaired glutamatergic regulation, followed by increased striatal dopamine release, aberrant salience, and psychotic symptoms. Acute psychosocial stress can activate increased striatal dopamine release both directly and indirectly via blunted cortical dopamine release and impaired glutamatergic regulation. The dopaminergic system interacts also with muscarinic cholinergic system and closely related muscarinic hypothesis of schizophrenia.
Glutamate hypotheses
The glutamate hypothesis assumes that schizophrenia is caused by developmental abnormalities in glutamate synapse formation at specific sites, particularly at GABA interneurons in the cerebral cortex. These abnormalities may lead to subsequent excessive glutamate signaling to the ventral tegmental area (VTA), and excessive activation of this pathway may result in an excess of dopamine in the ventral striatum via the mesolimbic pathway[54]. The role of dysregulation of glutamatergic neurotransmission in the pathophysiology of schizophrenia is supported by evidence from genetics, pharmacological, postmortem, and brain imaging studies[55]. The convergence of GABA impairment and glutamate neurotransmission in the dorsolateral prefrontal cortex could explain the impairment of certain cognitive functions in schizophrenia[56].
The NMDA receptor hypofunction hypothesis[57] assumes that genetic and other risk factors induce epigenetic alterations leading to NMDA receptor hypofunction in schizophrenia. NMDA receptor hypofunction induces a cascade of downstream disturbances in neuronal activity, calcium entry, and epigenetic machinery, leading to abnormal synaptic development and dopaminergic and GABAergic dysfunction. These changes in neurotransmission result in the cognitive and social deficits found in schizophrenia. According to this hypothesis, changes in the dopamine system are secondary to NMDA receptor hypofunction.
Antagonists of NMDA receptors (e.g., phencyclidine) have been shown to cause symptoms similar to the positive and negative symptoms and cognitive defects in schizophrenia[58]. According to increasing evidence, deficits in NMDA transmission are linked to cognitive defects and negative symptomatology[59].
Serotonin hypothesis
There are 3 interconnected pathways hypothetically associated with hallucinations and delusions: (1) Dopamine hyperactivity at D2 dopamine receptors in the mesolimbic pathway, which extends from the VTA to the ventral striatum; (2) NMDA receptor hypoactivity on GABAergic interneurons in the prefrontal cortex; and (3) Serotonin (5-HT) hyperactivity of 5-HT2A receptors on glutamate neurons in the cerebral cortex. All 3 pathways can lead to hyperactivity of the mesolimbic dopamine pathway[54].
According to the serotonin hypothesis[60], the basic cause of schizophrenia is stress-induced serotonergic hyperfunction in the cerebral cortex, especially in the anterior cingulate cortex and the dorsolateral frontal lobe. The serotonin hypothesis assumes hyperfunction of 5-HT2A receptors on glutamate neurons in the cerebral cortex. This overactivation of 5-HT2A receptors may be due to an excess of serotonin, upregulation of 5-HT2A receptors, or the effects of 5-HT2A receptor agonists. Subsequent release of glutamate in the VTA may activate the mesolimbic pathway, resulting in excess dopamine in the ventral striatum[54].
Cannabinoid hypothesis
According to the cannabinoid hypothesis[61-63], changes in the endocannabinoid system may contribute to the pathogenesis of schizophrenia. This hypothesis proposes that increased activation of the endocannabinoid system through CB1 receptors on GABAergic interneurons in the ventral tegmental area, basolateral amygdala, and medial prefrontal cortex may lead to a hyperdopaminergic and hypoglutamatergic status, which may cause schizophrenia. The hypothesis was supported by evidence that cannabis use in adolescence is an independent risk factor for schizophrenia development (OR: 3.90)[31] and by the confirmation of interactions between the cannabinoid and dopamine systems that may be related to the processes associated with drug addiction or schizophrenia[64].
BLOOD BRAIN BARRIER
The pathophysiology of many central nervous system (CNS) disorders, including schizophrenia, includes altered function of the blood brain barrier (BBB), as shown by evidence from neuroimaging studies, research of both cerebrospinal fluid (CSF) and blood-based biomarkers, and postmortem studies[65]. It remains to be elucidated whether BBB dysfunction is the cause or consequence of schizophrenia pathology[65]. P-glycoprotein is highly expressed in capillary endothelial cells. P-glycoprotein limits the accumulation of psychotropic drugs in the brain and is responsible for the efflux of drugs from the CNS by using the energy from ATP hydrolysis to return the compound to the bloodstream.
According to increasing evidence, malfunction of the BBB and microvascular abnormalities contribute to the pathophysiology of schizophrenia[66]. In a postmortem study, the cellular expression of ABCB1 [the gene encoding P-glycoprotein 1 (P-gp); multidrug resistance protein 1] was examined in patients with schizophrenia. A reduced density of P-gp-expressing neurons was found in the medial habenula of patients with schizophrenia compared to that of controls[66]. Furthermore, polymorphisms of ABCB1 have been associated with changes in drug disposition and pharmacotherapy response[67].
P-gp is not the only efflux protein; multiple drug resistance (MRP) and breast cancer resistance protein (BCRP) also facilitate the efflux of ATP-dependent substrates. In addition to P-gp, BCRP (ABCG2) and the multidrug resistance proteins MRP1 (ABCC1) and MRP2 (ABCC2) are ATP-dependent efflux transporters present in the BBB[68].
Claudin-5 is a component of tight junctions and is specifically expressed in endothelial cells in the CNS. Polymorphism of claudin-5 has been associated with schizophrenia risk[65], and serum claudin-5 Levels were decreased in patients with schizophrenia[69]. The expression of claudin-5 in the hippocampus was reduced in patients with schizophrenia; the levels of claudin-5 correlated with the duration and age of onset of the disease[70]. The BBB impedes the transfer of many drugs, including antipsychotics, as well as some inflammatory molecules, such as cytokines, which play an important role in the pathophysiology of schizophrenia (see below).
Other explanations of pharmacoresistance in schizophrenia involve abnormal structure of the BBB, downregulation of genes encoding ion transport proteins, impaired immune system, dysfunctional glutamatergic transmission, etc.
NEUROINFLAMMATION
Based on the observation that schizophrenia is often associated with chronic neuroinflammation in the CNS[71], the vulnerability-stress model has been expanded into the vulnerability-stress-inflammation model[72], which suggests that the symptoms of schizophrenia are associated with specific changes in dopaminergic, serotonergic, noradrenergic, and glutamatergic neurotransmission following neuroinflammation and microglial activation. The hypothesis is based on the following findings: (1) Stress can increase proinflammatory cytokines and may even contribute to a chronic proinflammatory condition; (2) The typical changes in neurotransmission observed in schizophrenia have also been found in low-level neuroinflammation; (3) Risk factors for schizophrenia include genes whose expression promotes inflammation, environmental stressors, alterations of the immune system, severe infections, and autoimmune disorders; and (4) Antipsychotics also provide anti-inflammatory and immunomodulatory effects.
The vulnerability-stress-inflammation model of schizophrenia suggests that genetic vulnerability and infection during pregnancy may induce a proinflammatory response in the mother, causing deleterious effects on the neurodevelopment of the fetus and increasing the risk of developing schizophrenia. The development of the glutamate system may be disrupted. Re-exposure to stress at a later age may be followed by increased cytokine release, astrocyte activation or loss, dopaminergic hyperactivity, and NMDA antagonism, leading to the positive, negative, and cognitive symptoms of schizophrenia. Immune conditioning and immune sensitization can elicit a repeated response to stress leading to the symptoms of the disease.
Immunologic processes in schizophrenia
Currently, the immune system, immunological processes and inflammation are believed to have a significant role in the neurobiology of schizophrenia[73]. Evidence of immune etiology in schizophrenia comes from GWAS, where a significant association between schizophrenia and the expression of MHC, located on chromosome 6, was observed[16,74].
The relationship between neurotransmitters and mediators of the inflammatory response can be reciprocal; an immunoregulatory function of dopamine has been described. Increased expression of dopamine D3 receptors and increased synthesis of interferon gamma (IFNγ) in lymphocytes were observed in nonmedicated patients suffering from schizophrenia[75]. An important finding from PET studies of inflammation with elevation of proinflammatory cytokines produced by microglia was an elevated microglial activity in subjects with subclinical symptoms and patients with schizophrenia[64].
Numerous studies have found immune dysregulation in patients with schizophrenia compared to healthy controls, and several meta-analyses have concluded that patients with schizophrenia exhibit signs of low-grade peripheral inflammation characterized by upregulated proinflammatory cytokines and acute phase proteins[76-78]. A recent meta-analysis of postmortem brain studies evaluating histological alterations of cellular composition and those assessing molecular parameters strengthened the immunologic hypothesis of schizophrenia[79]. The authors found significant increases in the density of microglia (especially in the temporal cortex) and the overall expression of pro-inflammatory genes but no difference in the expression of anti-inflammatory genes in patients with schizophrenia compared to those in controls. However, it is important to note that these immunological alterations have been found only in a subgroup of patients with schizophrenia: approximately 40% of studied patients have exhibited some level of inflammation[80,81]. As schizophrenia is seen as a syndrome consisting of several disease phenotypes with different underlying pathologies, it is crucial to define robust immune biomarkers that would help in the identification of patient groups that might benefit from anti-inflammatory therapy[76,82]. Cytokines represent a broad category of signaling molecules produced by a wide range of cells, including immune cells such as B and T lymphocytes, macrophages, and mastocytes, as well as endothelial cells and fibroblasts. A meta-analysis of 18 studies found alterations in both proinflammatory and anti-inflammatory cytokines, and these disturbances were stage dependent[83]. In patients with first-episode psychosis, elevated levels of proinflammatory cytokines were found, whereas the level of interleukin (IL)-4 was significantly reduced. In acutely ill patients, increased pro-inflammatory cytokines were observed, and lower levels of IL-4 and IL-10 Levels were found than in controls. In chronically ill patients, augmented levels of IL-1β, sIL-2R, IL-6, and tumor necrosis factor alpha (TNF-α) and reduced IFNγ levels were observed compared to those in controls. Details are summarized in Table 1.
Table 1.
Possible diagnostic/theranostic immunologic biomarkers in schizophrenia
|
Parameter
|
Serum/plasma/peripheral blood
|
CSF
|
| Pro-inflammatory cytokines | ↑ IL-6, IFN-γ, IL-1RA, IL-1β, IL-6, IL-8, IL-12, sIL-2R, TGF-β, and TNF-α | ↑ IL-1β, IL-6 and IL-8 |
| Anti-inflammatory cytokines | ↓ IL-10 and IL-4 | |
| Acute phase proteins | ↑ CRP, haptoglobin, α-1 antitrypsin, and α-2 macroglobulin | |
| Antibodies | ↑ Anti-cardiolipin IgG and anti-NMDA receptor titers | |
| Immune cells | ↑ CD4+, CD3+ and CD56+ | |
| Other biomolecules/metabolites | ↓ Creatine kinase m/B, MMP3, ACE, cortisol, TBG, α-2 macroblobulin, thrombopoietin, TSH, and ICAM-1, P-selectin |
ACE: Angiotensin-converting enzyme; CRP: C-reactive protein; CSF: Cerebro-spinal fluid; ICAM: Intercellular adhesion molecule; IFNγ: Interferon gamma; IL: Interleukin; MMP3: Matrix metalloproteinase 3; NMDA: N-methyl-D-aspartate; TBG: Thyroxine-binding globulin; TGF-β: Transforming growth factor-beta; TNF-α: Tumor necrosis factor-alpha; TSH: Thyroid-stimulating hormone.
Moreover, a study evaluating the gene expression of cytokines in peripheral blood mononuclear cells reported increased mRNA levels of IL-6, IL-8 and TNF-α and decreased anti-inflammatory IL-2 mRNA[81]. Alterations in these cytokines were also found in CFS[84], and a meta-analysis of 16 studies found significantly higher CSF levels of IL-1β, IL-6 and IL-8 in patients with schizophrenia compared to healthy controls. Interleukins (e.g., IL-1β and IL-6) play important roles in neurotransmitter systems in schizophrenia. A relationship exists between increased concentrations of IL-6 in childhood and a higher risk of subclinical psychotic symptoms in young adulthood. Increased concentrations of IL-6 and other proinflammatory cytokines, such as TNF-α, IL-1β, and IFNγ, are normalized in episodes of remission after antipsychotic treatment. Some studies have suggested an association among increased serum concentrations of cytokines, including IL-6, severity of the disease, and duration and antipsychotic therapy[74].
Alterations in the immune system influence the neurotransmission of dopamine, 5-HT, norepinephrine, and glutamate. The immune system can activate indoleamine 2,3-dioxygenase, an enzyme involved in tryptophan/kynurenine metabolism[73]. Kynurenic acid acts as a naturally occurring NMDA antagonist in the human brain. Increased levels of kynurenic acid were found in the CSF of patients with schizophrenia[85,86]; however, no changes in kynurenic acid levels were observed in the peripheral blood of patients with schizophrenia[73]. Proinflammatory cytokines increase the concentration of kynurenic acid. Approximately 10% of nonmedicated patients in acute episodes of schizophrenia produce NMDA receptor antibodies, and this finding supports the hypothesis of NMDA receptor antagonism in schizophrenia[72,73].
C-reactive protein (CRP) appears to be the most promising theranostic marker for inflammation, and patients with increased CRP might benefit from anti-inflammatory therapy[76,87]. CRP is synthesized in the liver in response to IL-1β, IL-6 and TNF-α and is released from macrophages and adipocytes[76]. CRP has been reported to correlate both with positive and negative symptoms of schizophrenia[88] and with cognitive dysfunction[89]. Recently, “ultraresistance” to treatment in schizophrenia (defined as current clozapine treatment and a mean positive and negative syndrome scale (PANSS) score ≥ 70) was found to be associated with abnormal CRP levels (> 3 g/L), providing further justification for treating ultra-resistant patients with anti-inflammatory agents[90]. Additionally, other acute phase proteins, including haptoglobin, alpha-1 antitrypsin, and alpha-2-macroglobulin, were also found to be elevated in a subgroup of individuals with schizophrenia and other psychoses[91,92].
Additional biomolecules and metabolites essential for inflammation and endothelial cell function (e.g., creatine kinase m/B, angiotensin-converting enzyme, matrix metalloproteinase, thyroid-stimulating hormone, thyroxine-binding globulin, intercellular adhesion molecule 1, cortisol, α-2-macroglobulin, and thrombopoietin) have been found in lower concentrations in drug-naïve patients than in controls[93]. The authors commented that most of these proteins are involved. Additionally, upregulation of leucocyte adhesion molecules was described in psychotic disorders, increased levels of soluble L-selectin were detected in the serum of drug-naïve patients with schizophrenia, and the serum levels of L-selectin and P-selectin in patients with schizophrenia did not differ from those in healthy controls[94]. In another study, P-selectin plasma levels were found to be increased in patients with acute psychosis[95].
Certain studies have also found changes in the number of immune cells in patients with schizophrenia compared to healthy controls. A meta-analysis of 16 studies evaluating blood lymphocyte counts found a significant increase in the percentages of CD4+ (T-helper lymphocytes) and CD56+ (natural killer cells) lymphocytes in acutely relapsed inpatients and a significant increase in the absolute numbers of total lymphocytes and CD3+ (T-lymphocytes) and CD4+ cells[96]. However, a significant decrease in the percentage of CD3+ cells was found in drug-naïve patients in the first episode of schizophrenia. Additionally, some autoimmune responses were also found in schizophrenic patients, but their clinical relevance remains elusive. Regarding the pathogenesis of schizophrenia, different autoantibodies, as well as antibodies against diet antigens, e.g., gliadin and casein, were investigated in different parts of the brain, serum, and CSF. A systematic quantitative review of 81 studies found significantly increased anti-cardiolipin IgG and anti-NMDA receptor autoantibody titers in patients in the first episode of schizophrenia[97]. The authors also reported increased titers of anti-cardiolipin IgG and IgM and nerve growth factor in patients with schizophrenia compared with controls.
As mentioned, there is a strong link between oxidative stress and the immune system; therefore, by counteracting oxidative stress, antioxidants reduce inflammation and the overactive immune response. Glutathione is an antioxidant that is essential in the myelinization and maturation of white matter and can be supplemented as the amino acid precursor N-acetyl cysteine. N-acetyl cysteine possesses antioxidant properties and mild anti-inflammatory effects and regulates synaptic NMDA receptors. NAC supplementation for 6 mo ameliorated positive symptoms and improved neurocognition (processing speed) in patients with schizophrenia with high peripheral oxidative stress[98]. Omega-3-type polyunsaturated fatty acids have also exerted antioxidative capacity and anti-inflammatory effects[99].
Non-steroidal anti-inflammatory drugs in pharmacotherapy
Anti-inflammatory agents have shown some benefits as adds-ons to antipsychotic treatment in schizophrenia, as reported by two recent meta-analyses[100,101]. A meta-analysis of 62 double-blind randomized clinical trials studying aspirin, celecoxib, omega-3 fatty acids, estrogens, pregnenolone, minocycline, N-acetyl cysteine, and erythropoietin in 2914 patients found an overall significant effect of a decrease in the PANSS score[100]. Additionally, cognitive improvement was significantly associated with minocycline and pregnenolone therapy. Another meta-analysis of 70 randomized clinical trials including 4104 subjects investigated either primarily non-steroidal anti-inflammatory drugs (NSAIDs), minocycline and monoclonal antibodies, or drugs with potential anti-inflammatory properties (N-acetyl cysteine, melatonin, neurosteroids, estrogens, fatty acids, statins, and glitazones) as adjunctive therapies to antipsychotics. The analysis also found a decrease in the PANSS score[101]. Small but significant effect sizes were observed on both negative and positive symptoms, general psychopathology and working memory. Interestingly, primarily anti-inflammatory drugs were not found to be superior to potential anti-inflammatory drugs. However, the authors highlighted that the reported effects might be overestimated due to the many small study samples included in the analysis.
In conclusion, changes in the frequencies of immune cells, the levels and expression of cytokines, and the levels of acute phase proteins in the blood and CSF were observed in patients with schizophrenia compared to healthy controls, and CRP seems to be a promising theranostic biomarker for schizophrenia. Moreover, larger studies with longer treatment durations and the inclusion of only schizophrenic patients with proven inflammation (CRP levels > 3 g/L) are warranted to elucidate the efficacy of anti-inflammatory treatment in schizophrenia.
The challenges of drug development include the development of novel molecules affecting the immune system and of immunotherapy using autoantibodies as well as the stratification of patients with schizophrenia according to their immune phenotypes to enable the selection of effective pharmacotherapeutic agents[74].
MICROBIOME-BRAIN AXIS MODEL
The microbiome-gut-brain axis model postulates that bidirectional communication between the central and enteric nervous systems ensures the connection of the brain with peripheral intestinal functions[102,103]. It is presumed that the gut microbiome can program brain function during early development; in other words, active signals from the microbiome play a critical role in brain development. It is thought that the microbiome may affect brain development through epigenetic mechanisms[104]. Signal pathways from the gut microbiome to the brain include: (1) The direct activation of the vagus nerve; (2) The production or induction of various metabolites that may cross the BBB to regulate neurological functions; and (3) An immune system whose cytokines affect neurophysiology[105].
Probiotics modulate the immune responses of the host and could be beneficial for schizophrenia patients[106]. The immunomodulatory effects of probiotic supplementation were examined after 14 wk in chronic patients with schizophrenia, and Lactobacillus and Bifidobacterium were administered as adjuvant treatments. Increased levels of brain-derived neurotrophic factor, chemokine ligand 5 (RANTES), monocyte chemotactic protein and macrophage inflammatory protein-1 beta were found[106]. Gut-brain communication suggests the direct secretion of some neuroactive substances, and some intestinal bacteria can produce mediators, e.g., GABA, acetylcholine.
INNOVATIVE DRUG APPROACHES AND TARGETS
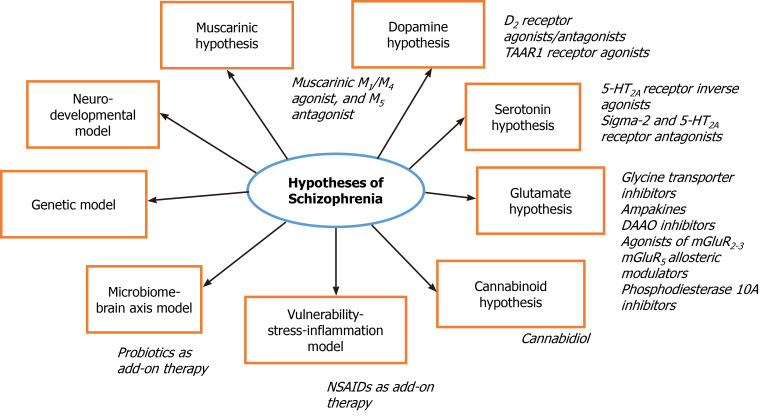
All antipsychotics for schizophrenia treatment are based on dopamine agonism/ antagonism, and no nondopaminergic antipsychotics have yet been developed or approved (Figure 1). Lumateperone is characterized as a partial D2 receptor agonist/antagonist and a 5-HT2A antagonist, and it is a serotonin reuptake inhibitor that indirectly modulates glutamatergic transmission (effect on NMDA receptor subtype 2B, NR2B)[107,108]. It has no metabolic or extrapyramidal adverse effects and positively affects cognition[109]. Another study found that lumateperone was well tolerated with minimal extrapyramidal and cardiometabolic adverse effects and maintained or reduced the symptoms of schizophrenia after a switch from a previous antipsychotic[110].
Figure 1.
Illustration of current hypotheses and novel approaches in treatment of schizophrenia. DAAO: D-Amino acid oxidase; TAAR1: Trace amine-associated receptor 1; NSAIDs: Non-steroidal anti-inflammatory drugs.
A promising therapeutic drug strategy for schizophrenia is the use of antipsychotics without D2 receptor binding[111]. Agonists of trace amine-associated receptor 1 (TAAR1) seem to selectively affect dopamine and may represent a new class of psychotropic drugs[112,113]. A novel compound, SEP-363856, was described as a TAAR1 and 5-TH1A agonist and was tested in a pilot trial with 120 patients with an acute exacerbation of schizophrenia. The reduction in the PANSS score of the treatment group was significant compared with that of the placebo group; longer and larger trials are necessary to prove the efficacy and safety of this TAAR1 agonist[113]. Other TAAR1 agonists, e.g., RO5263397, have been developed and are being tested for use in schizophrenia. RO5263397 was found to be safe, but a great variety in metabolism and plasma levels was found that depended on ethnicity and genotype[112,114].
Novel drugs indirectly targeting glutamate neurotransmission are among the strategies being pursued for antipsychotic development. Inhibitors of glycine transporters (e.g., sarcosine and bitopertin), coadministration of NMDA agonists, allosteric modulators of AMPA receptors (ampakines), and allosteric modulators of mGluR5 are being tested; novel drugs affecting the glutamate ionotropic and metabotropic receptors mGluR2-3 have been developed[115].
Agonists of mGluR2-3 have anxiolytic properties and reverse the effects of stress; agonists of the mGluR3 receptor antagonize the effect of phencyclidine and amphetamine. Novel drugs directly affecting receptors were found to be ineffective and/or to have adverse effects. Pomaglumetad methionil is an mGluR2-3 agonist[58]. It was tested as an adjunctive therapy for patients with negative symptoms of schizophrenia, and the study did not find a difference between the pomaglumetad group and the placebo group[116]. Post hoc analyses suggested the efficacy of pomaglumetad in patients suffering from schizophrenia for less than or equal to 3 years or in patients previously treated with antipsychotics predominantly acting as D2 antagonists[117,118]. Thus, the potential of novel mGluR2-3 agonists is suggested for treating psychosis and aggression/agitation associated with neurodegenerative diseases (Parkinson’s disease, Alzheimer’s disease, dementia with Lewy bodies, etc.)[117].
D-Amino acid oxidase (DAAO) inhibitors were found to modulate NMDA transmission. Sodium benzoate, a DAAO inhibitor, has been tested as an add-on therapy and improved symptoms in clozapine-resistant patients[119]. In another study, sodium benzoate was administered to patients with schizophrenia as an add-on therapy compared to placebo. The adjunctive therapy was well tolerated, and chronic schizophrenia patients showed improved function, especially neurocognition[120].
Cannabidiol seems to be a promising candidate for the treatment of schizophrenia. Increased function of the endocannabinoid system was observed in schizophrenic patients; cannabidiol was found to decrease mesolimbic dopaminergic activity. There is evidence that chronic and acute administration of cannabidiol led to improvement of schizophrenia symptomatology[121].
Based on the serotonin hypothesis, pimavanserin was developed and characterized as an inverse agonist at 5-HT2A receptors, and it has binding affinity for sigma-1 receptors. This antipsychotic has been FDA-approved for the treatment of psychosis associated with Parkinson’s disease. Further randomized controlled trials are needed to consider pimavanserin as a drug for schizophrenia treatment[122].
Antagonism at sigma-2 receptors was described for roluperidone, which was developed as a novel antipsychotic affecting 5-HT2A receptors and sigma-2 receptors[58]. Roluperidone has been found to be effective in the treatment of negative symptoms[123].
Xanomeline is a muscarinic M1/M4 agonist and M5 antagonist that were originally developed for Alzheimer´s disease. Currently, it is being tested in combination with trospium, which reduces peripheral adverse effects (nausea and vomiting), as a new antipsychotic with a novel mechanism of action.
Phosphodiesterase inhibitors have been investigated as drugs to enhance cognition in schizophrenia[124]. Phosphodiesterase 10A inhibitors likely modulate D1 (directly) and D2 (indirectly) striatal pathways and regulate glutamate receptors[112]. TAK-063, a phosphodiesterase 10A inhibitor, was tested, but the clinical trial did not meet the primary endpoint, and extrapyramidal syndromes occurred more often in the TAK-063 group than in the placebo group[125].
CONCLUSION
Genetic predisposition and neurodevelopmental and environmental risk factors for schizophrenia were summarized. Nevertheless, the understanding of schizophrenia pathophysiology is limited, and current pharmacotherapy is complicated by adverse effects, pharmacoresistance, and low compliance of patients. Novel targets and approaches of antipsychotic treatment are being developed with the aim of covering the wide range of schizophrenia symptoms, especially negative symptomatology, cognitive impairment, and residual and treatment-resistant symptoms. Novel drug targets based on current schizophrenia hypotheses include molecules indirectly targeting glutamate neurotransmission, DAAO inhibitors, 5-HT2A receptor inverse agonists, sigma-2 receptor antagonists, phosphodiesterase inhibitors, etc. In pharmacoresistant patients, possible comorbidities may be related to inflammation or a disrupted microbiome-gut-brain axis, and augmentation of antipsychotic treatment via NSAID or probiotic administration can be considered. Further research on schizophrenia pathophysiology, genetic predisposition (based on GWAS), regulatory mechanisms in impaired mediator transmission and other factors is needed to improve the clinical outcomes of pharmacotherapy.
Footnotes
Conflict-of-interest statement: Authors declare that there is no conflict of interest regarding the publication of this article.
Manuscript source: Invited manuscript
Peer-review started: February 27, 2021
First decision: March 30, 2021
Article in press: June 18, 2021
Specialty type: Psychiatry
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Y S-Editor: Zhang H L-Editor: A P-Editor: Xing YX
Contributor Information
Matej Ľupták, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague 12800, Czech Republic.
Danica Michaličková, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague 12800, Czech Republic.
Zdeněk Fišar, Department of Psychiatry, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague 12000, Czech Republic.
Eva Kitzlerová, Department of Psychiatry, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague 12000, Czech Republic.
Jana Hroudová, Institute of Pharmacology, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague 12800, Czech Republic; Department of Psychiatry, First Faculty of Medicine, Charles University and General University Hospital in Prague, Prague 12000, Czech Republic. hroudova.jana@gmail.com.
References
- 1.Ortiz-Orendain J, Castiello-de Obeso S, Colunga-Lozano LE, Hu Y, Maayan N, Adams CE. Antipsychotic combinations for schizophrenia. Cochrane Database Syst Rev . 2017;6:CD009005. doi: 10.1002/14651858.CD009005.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull . 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bebawy M, Chetty M. Differential pharmacological regulation of drug efflux and pharmacoresistant schizophrenia. Bioessays . 2008;30:183–188. doi: 10.1002/bies.20706. [DOI] [PubMed] [Google Scholar]
- 4.Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res . 2012;138:18–28. doi: 10.1016/j.schres.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordström AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry . 1993;33:227–235. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- 6.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry . 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 7.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol . 2015;29:97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai CY, Scarr E, Udawela M, Everall I, Chen WJ, Dean B. Biomarkers in schizophrenia: A focus on blood based diagnostics and theranostics. World J Psychiatry . 2016;6:102–117. doi: 10.5498/wjp.v6.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blokhin IO, Khorkova O, Saveanu RV, Wahlestedt C. Molecular mechanisms of psychiatric diseases. Neurobiol Dis . 2020;146:105136. doi: 10.1016/j.nbd.2020.105136. [DOI] [PubMed] [Google Scholar]
- 10.Brum CB, Paixão-Côrtes VR, Carvalho AM, Martins-Silva T, Carpena MX, Ulguim KF, Luquez KYS, Salatino-Oliveira A, Tovo-Rodrigues L. Genetic variants in miRNAs differentially expressed during brain development and their relevance to psychiatric disorders susceptibility. World J Biol Psychiatry . 2020:1–12. doi: 10.1080/15622975.2020.1834618. [DOI] [PubMed] [Google Scholar]
- 11.McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-An Overview. JAMA Psychiatry . 2020;77:201–210. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 12.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet . 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howes OD, McCutcheon R, Owen MJ, Murray RM. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol Psychiatry . 2017;81:9–20. doi: 10.1016/j.biopsych.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron . 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Comer AL, Carrier M, Tremblay MÈ, Cruz-Martín A. The Inflamed Brain in Schizophrenia: The Convergence of Genetic and Environmental Risk Factors That Lead to Uncontrolled Neuroinflammation. Front Cell Neurosci . 2020;14:274. doi: 10.3389/fncel.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Crowe RR, Oksenberg JR, Mirel DB, Kendler KS, Freedman R, Gejman PV. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature . 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tost H, Callicott JH, Rasetti R, Vakkalanka R, Mattay VS, Weinberger DR, Law AJ. Effects of neuregulin 3 genotype on human prefrontal cortex physiology. J Neurosci . 2014;34:1051–1056. doi: 10.1523/JNEUROSCI.3496-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Pino I, García-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martínez de Lagrán M, Ciceri G, Gabaldón MV, Moratal D, Dierssen M, Canals S, Marín O, Rico B. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron . 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Fazzari P, Paternain AV, Valiente M, Pla R, Luján R, Lloyd K, Lerma J, Marín O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature . 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 20.Callicott JH, Feighery EL, Mattay VS, White MG, Chen Q, Baranger DA, Berman KF, Lu B, Song H, Ming GL, Weinberger DR. DISC1 and SLC12A2 interaction affects human hippocampal function and connectivity. J Clin Invest . 2013;123:2961–2964. doi: 10.1172/JCI67510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wexler EM, Geschwind DH. DISC1: a schizophrenia gene with multiple personalities. Neuron . 2011;72:501–503. doi: 10.1016/j.neuron.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Kang E, Burdick KE, Kim JY, Duan X, Guo JU, Sailor KA, Jung DE, Ganesan S, Choi S, Pradhan D, Lu B, Avramopoulos D, Christian K, Malhotra AK, Song H, Ming GL. Interaction between FEZ1 and DISC1 in regulation of neuronal development and risk for schizophrenia. Neuron . 2011;72:559–571. doi: 10.1016/j.neuron.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houenou J, Boisgontier J, Henrion A, d'Albis MA, Dumaine A, Linke J, Wessa M, Daban C, Hamdani N, Delavest M, Llorca PM, Lançon C, Schürhoff F, Szöke A, Le Corvoisier P, Barau C, Poupon C, Etain B, Leboyer M, Jamain S. A Multilevel Functional Study of a SNAP25 At-Risk Variant for Bipolar Disorder and Schizophrenia. J Neurosci . 2017;37:10389–10397. doi: 10.1523/JNEUROSCI.1040-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badowska DM, Brzózka MM, Kannaiyan N, Thomas C, Dibaj P, Chowdhury A, Steffens H, Turck CW, Falkai P, Schmitt A, Papiol S, Scheuss V, Willig KI, Martins-de-Souza D, Rhee JS, Malzahn D, Rossner MJ. Modulation of cognition and neuronal plasticity in gain- and loss-of-function mouse models of the schizophrenia risk gene Tcf4. Transl Psychiatry . 2020;10:343. doi: 10.1038/s41398-020-01026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenwood TA, Lazzeroni LC, Maihofer AX, Swerdlow NR, Calkins ME, Freedman R, Green MF, Light GA, Nievergelt CM, Nuechterlein KH, Radant AD, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Gur RC, Gur RE, Braff DL. Genome-wide Association of Endophenotypes for Schizophrenia From the Consortium on the Genetics of Schizophrenia (COGS) Study. JAMA Psychiatry . 2019;76:1274–1284. doi: 10.1001/jamapsychiatry.2019.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet . 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fromer M, Roussos P, Sieberts SK, Johnson JS, Kavanagh DH, Perumal TM, Ruderfer DM, Oh EC, Topol A, Shah HR, Klei LL, Kramer R, Pinto D, Gümüş ZH, Cicek AE, Dang KK, Browne A, Lu C, Xie L, Readhead B, Stahl EA, Xiao J, Parvizi M, Hamamsy T, Fullard JF, Wang YC, Mahajan MC, Derry JM, Dudley JT, Hemby SE, Logsdon BA, Talbot K, Raj T, Bennett DA, De Jager PL, Zhu J, Zhang B, Sullivan PF, Chess A, Purcell SM, Shinobu LA, Mangravite LM, Toyoshiba H, Gur RE, Hahn CG, Lewis DA, Haroutunian V, Peters MA, Lipska BK, Buxbaum JD, Schadt EE, Hirai K, Roeder K, Brennand KJ, Katsanis N, Domenici E, Devlin B, Sklar P. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci . 2016;19:1442–1453. doi: 10.1038/nn.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry . 2020;7:272–281. doi: 10.1016/S2215-0366(19)30302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophr Res . 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Fatemi SH, Folsom TD, Rooney RJ, Mori S, Kornfield TE, Reutiman TJ, Kneeland RE, Liesch SB, Hua K, Hsu J, Patel DH. The viral theory of schizophrenia revisited: abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology . 2012;62:1290–1298. doi: 10.1016/j.neuropharm.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belbasis L, Köhler CA, Stefanis N, Stubbs B, van Os J, Vieta E, Seeman MV, Arango C, Carvalho AF, Evangelou E. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand . 2018;137:88–97. doi: 10.1111/acps.12847. [DOI] [PubMed] [Google Scholar]
- 32.Davies C, Segre G, Estradé A, Radua J, De Micheli A, Provenzani U, Oliver D, Salazar de Pablo G, Ramella-Cravaro V, Besozzi M, Dazzan P, Miele M, Caputo G, Spallarossa C, Crossland G, Ilyas A, Spada G, Politi P, Murray RM, McGuire P, Fusar-Poli P. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet Psychiatry . 2020;7:399–410. doi: 10.1016/S2215-0366(20)30057-2. [DOI] [PubMed] [Google Scholar]
- 33.Kneeland RE, Fatemi SH. Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry . 2013;42:35–48. doi: 10.1016/j.pnpbp.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol . 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zubin J, Spring B. Vulnerability--a new view of schizophrenia. J Abnorm Psychol . 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]
- 36.Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci . 2016;17:125–134. doi: 10.1038/nrn.2015.19. [DOI] [PubMed] [Google Scholar]
- 37.Madireddy S, Madireddy S. Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia. Brain Sci . 2020;10 doi: 10.3390/brainsci10100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry . 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW. The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann Med . 2003;35:86–93. doi: 10.1080/07853890310010005. [DOI] [PubMed] [Google Scholar]
- 40.Piper M, Beneyto M, Burne TH, Eyles DW, Lewis DA, McGrath JJ. The neurodevelopmental hypothesis of schizophrenia: convergent clues from epidemiology and neuropathology. Psychiatr Clin North Am . 2012;35:571–584. doi: 10.1016/j.psc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl . 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 42.Friston KJ. The disconnection hypothesis. Schizophr Res . 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 43.Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res . 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 44.Keshavan MS, Mehta UM, Padmanabhan JL, Shah JL. Dysplasticity, metaplasticity, and schizophrenia: Implications for risk, illness, and novel interventions. Dev Psychopathol . 2015;27:615–635. doi: 10.1017/S095457941500019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, Debnath M, McGrath J, Maes M, Amminger P, McGorry PD, Pantelis C, Berk M. A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Neurosci Biobehav Rev . 2016;65:185–194. doi: 10.1016/j.neubiorev.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collin G, Keshavan MS. Connectome development and a novel extension to the neurodevelopmental model of schizophrenia. Dialogues Clin Neurosci . 2018;20:101–111. doi: 10.31887/DCNS.2018.20.2/gcollin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maas DA, Vallès A, Martens GJM. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry . 2017;7:e1171. doi: 10.1038/tp.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Shachar D. The bimodal mechanism of interaction between dopamine and mitochondria as reflected in Parkinson's disease and in schizophrenia. J Neural Transm (Vienna) . 2020;127:159–168. doi: 10.1007/s00702-019-02120-x. [DOI] [PubMed] [Google Scholar]
- 49.Jarskog LF. Apoptosis in schizophrenia: pathophysiologic and therapeutic considerations. Curr Opin Psychiatry . 2006;19:307–312. doi: 10.1097/01.yco.0000218603.25346.8f. [DOI] [PubMed] [Google Scholar]
- 50.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull . 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 51.Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology . 1988;1:179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 52.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry . 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 53.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull . 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr . 2018;23:187–191. doi: 10.1017/S1092852918001013. [DOI] [PubMed] [Google Scholar]
- 55.Uno Y, Coyle JT. Glutamate hypothesis in schizophrenia. Psychiatry Clin Neurosci . 2019;73:204–215. doi: 10.1111/pcn.12823. [DOI] [PubMed] [Google Scholar]
- 56.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol . 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 57.Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci . 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kantrowitz JT. Targeting Serotonin 5-HT2A Receptors to Better Treat Schizophrenia: Rationale and Current Approaches. CNS Drugs . 2020;34:947–959. doi: 10.1007/s40263-020-00752-2. [DOI] [PubMed] [Google Scholar]
- 59.Sershen H, Hashim A, Dunlop DS, Suckow RF, Cooper TB, Javitt DC. Modulating NMDA Receptor Function with D-Amino Acid Oxidase Inhibitors: Understanding Functional Activity in PCP-Treated Mouse Model. Neurochem Res . 2016;41:398–408. doi: 10.1007/s11064-016-1838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eggers AE. A serotonin hypothesis of schizophrenia. Med Hypotheses . 2013;80:791–794. doi: 10.1016/j.mehy.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Emrich HM, Leweke FM, Schneider U. Towards a cannabinoid hypothesis of schizophrenia: cognitive impairments due to dysregulation of the endogenous cannabinoid system. Pharmacol Biochem Behav . 1997;56:803–807. doi: 10.1016/s0091-3057(96)00426-1. [DOI] [PubMed] [Google Scholar]
- 62.El Khoury MA, Gorgievski V, Moutsimilli L, Giros B, Tzavara ET. Interactions between the cannabinoid and dopaminergic systems: evidence from animal studies. Prog Neuropsychopharmacol Biol Psychiatry . 2012;38:36–50. doi: 10.1016/j.pnpbp.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Müller-Vahl KR, Emrich HM. Cannabis and schizophrenia: towards a cannabinoid hypothesis of schizophrenia. Expert Rev Neurother . 2008;8:1037–1048. doi: 10.1586/14737175.8.7.1037. [DOI] [PubMed] [Google Scholar]
- 64.Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V, Howes OD. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry . 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry . 2018;5:79–92. doi: 10.1016/S2215-0366(17)30293-6. [DOI] [PubMed] [Google Scholar]
- 66.Bernstein HG, Hildebrandt J, Dobrowolny H, Steiner J, Bogerts B, Pahnke J. Morphometric analysis of the cerebral expression of ATP-binding cassette transporter protein ABCB1 in chronic schizophrenia: Circumscribed deficits in the habenula. Schizophr Res . 2016;177:52–58. doi: 10.1016/j.schres.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 67.Wolking S, Schaeffeler E, Lerche H, Schwab M, Nies AT. Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-Glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature. Clin Pharmacokinet . 2015;54:709–735. doi: 10.1007/s40262-015-0267-1. [DOI] [PubMed] [Google Scholar]
- 68.Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci . 2006;8:311–321. doi: 10.31887/DCNS.2006.8.3/fgirardin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Usta A, Kılıç F, Demirdaş A, Işık Ü, Doğuç DK, Bozkurt M. Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci . 2021;271:767–773. doi: 10.1007/s00406-020-01152-9. [DOI] [PubMed] [Google Scholar]
- 70.Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry . 2020;10:373. doi: 10.1038/s41398-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson G, Maes M, Berk M. Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog Neuropsychopharmacol Biol Psychiatry . 2013;42:101–114. doi: 10.1016/j.pnpbp.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Müller N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr Bull . 2018;44:973–982. doi: 10.1093/schbul/sby024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci . 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry . 2015;2:258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, Fuchs S. A peripheral marker for schizophrenia: Increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci USA . 2001;98:625–628. doi: 10.1073/pnas.021535398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kroken RA, Sommer IE, Steen VM, Dieset I, Johnsen E. Constructing the Immune Signature of Schizophrenia for Clinical Use and Research; An Integrative Review Translating Descriptives Into Diagnostics. Front Psychiatry . 2018;9:753. doi: 10.3389/fpsyt.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prins BP, Abbasi A, Wong A, Vaez A, Nolte I, Franceschini N, Stuart PE, Guterriez Achury J, Mistry V, Bradfield JP, Valdes AM, Bras J, Shatunov A PAGE Consortium; International Stroke Genetics Consortium; Systemic Sclerosis consortium; Treat OA consortium; DIAGRAM Consortium; CARDIoGRAMplusC4D Consortium; ALS consortium; International Parkinson’s Disease Genomics Consortium; Autism Spectrum Disorder Working Group of the Psychiatric Genomics Consortium; CKDGen consortium; GERAD1 Consortium; International Consortium for Blood Pressure; Schizophrenia Working Group of the Psychiatric Genomics Consortium; Inflammation Working Group of the CHARGE Consortium. Lu C, Han B, Raychaudhuri S, Bevan S, Mayes MD, Tsoi LC, Evangelou E, Nair RP, Grant SF, Polychronakos C, Radstake TR, van Heel DA, Dunstan ML, Wood NW, Al-Chalabi A, Dehghan A, Hakonarson H, Markus HS, Elder JT, Knight J, Arking DE, Spector TD, Koeleman BP, van Duijn CM, Martin J, Morris AP, Weersma RK, Wijmenga C, Munroe PB, Perry JR, Pouget JG, Jamshidi Y, Snieder H, Alizadeh BZ. Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study. PLoS Med . 2016;13:e1001976. doi: 10.1371/journal.pmed.1001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodrigues-Amorim D, Rivera-Baltanás T, Spuch C, Caruncho HJ, González-Fernandez Á, Olivares JM, Agís-Balboa RC. Cytokines dysregulation in schizophrenia: A systematic review of psychoneuroimmune relationship. Schizophr Res . 2018;197:19–33. doi: 10.1016/j.schres.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 79.van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry . 2017;7:e1075. doi: 10.1038/tp.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osimo EF, Cardinal RN, Jones PB, Khandaker GM. Prevalence and correlates of low-grade systemic inflammation in adult psychiatric inpatients: An electronic health record-based study. Psychoneuroendocrinology . 2018;91:226–234. doi: 10.1016/j.psyneuen.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boerrigter D, Weickert TW, Lenroot R, O'Donnell M, Galletly C, Liu D, Burgess M, Cadiz R, Jacomb I, Catts VS, Fillman SG, Weickert CS. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation . 2017;14:188. doi: 10.1186/s12974-017-0962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fond G, Lançon C, Korchia T, Auquier P, Boyer L. The Role of Inflammation in the Treatment of Schizophrenia. Front Psychiatry . 2020;11:160. doi: 10.3389/fpsyt.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry . 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang AK, Miller BJ. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr Bull . 2018;44:75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett . 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 86.Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull . 2012;38:426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z, Li P, Chi D, Wu T, Mei Z, Cui G. Association between C-reactive protein and risk of schizophrenia: An updated meta-analysis. Oncotarget . 2017;8:75445–75454. doi: 10.18632/oncotarget.17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liemburg EJ, Nolte IM PHAMOUS investigators. Klein HC, Knegtering H. Relation of inflammatory markers with symptoms of psychotic disorders: a large cohort study. Prog Neuropsychopharmacol Biol Psychiatry . 2018;86:89–94. doi: 10.1016/j.pnpbp.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Johnsen E, Fathian F, Kroken RA, Steen VM, Jørgensen HA, Gjestad R, Løberg EM. The serum level of C-reactive protein (CRP) is associated with cognitive performance in acute phase psychosis. BMC Psychiatry . 2016;16:60. doi: 10.1186/s12888-016-0769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fond G, Godin O, Boyer L, Berna F, Andrianarisoa M, Coulon N, Brunel L, Bulzacka E, Aouizerate B, Capdevielle D, Chereau I, D'Amato T, Dubertret C, Dubreucq J, Faget C, Leignier S, Lançon C, Mallet J, Misdrahi D, Passerieux C, Rey R, Schandrin A, Urbach M, Vidailhet P, Llorca PM, Schürhoff F, Leboyer M FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) Group. Chronic low-grade peripheral inflammation is associated with ultra resistant schizophrenia. Results from the FACE-SZ cohort. Eur Arch Psychiatry Clin Neurosci . 2019;269:985–992. doi: 10.1007/s00406-018-0908-0. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y, Wan C, Li H, Zhu H, La Y, Xi Z, Chen Y, Jiang L, Feng G, He L. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal Chem . 2006;78:3571–3576. doi: 10.1021/ac051916x. [DOI] [PubMed] [Google Scholar]
- 92.Yee JY, Nurjono M, Ng WY, Teo SR, Lee TS, Lee J. Peripheral blood gene expression of acute phase proteins in people with first episode psychosis. Brain Behav Immun . 2017;65:337–341. doi: 10.1016/j.bbi.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 93.Herberth M, Rahmoune H, Schwarz E, Koethe D, Harris LW, Kranaster L, Witt SH, Spain M, Barnes A, Schmolz M, Leweke MF, Guest PC, Bahn S. Identification of a molecular profile associated with immune status in first-onset schizophrenia patients. Clin Schizophr Relat Psychoses . 2014;7:207–215. doi: 10.3371/CSRP.HERA.020113. [DOI] [PubMed] [Google Scholar]
- 94.Iwata Y, Suzuki K, Nakamura K, Matsuzaki H, Sekine Y, Tsuchiya KJ, Sugihara G, Kawai M, Minabe Y, Takei N, Mori N. Increased levels of serum soluble L-selectin in unmedicated patients with schizophrenia. Schizophr Res . 2007;89:154–160. doi: 10.1016/j.schres.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 95.Masopust J, Malý R, Andrýs C, Vališ M, Bažant J, Hosák L. Markers of thrombogenesis are activated in unmedicated patients with acute psychosis: a matched case control study. BMC Psychiatry . 2011;11:2. doi: 10.1186/1471-244X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry . 2013;73:993–999. doi: 10.1016/j.biopsych.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ezeoke A, Mellor A, Buckley P, Miller B. A systematic, quantitative review of blood autoantibodies in schizophrenia. Schizophr Res . 2013;150:245–251. doi: 10.1016/j.schres.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 98.Conus P, Seidman LJ, Fournier M, Xin L, Cleusix M, Baumann PS, Ferrari C, Cousins A, Alameda L, Gholam-Rezaee M, Golay P, Jenni R, Woo TW, Keshavan MS, Eap CB, Wojcik J, Cuenod M, Buclin T, Gruetter R, Do KQ. N-acetylcysteine in a Double-Blind Randomized Placebo-Controlled Trial: Toward Biomarker-Guided Treatment in Early Psychosis. Schizophr Bull . 2018;44:317–327. doi: 10.1093/schbul/sbx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sommer IE, Bearden CE, van Dellen E, Breetvelt EJ, Duijff SN, Maijer K, van Amelsvoort T, de Haan L, Gur RE, Arango C, Díaz-Caneja CM, Vinkers CH, Vorstman JA. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr . 2016;2:16003. doi: 10.1038/npjschz.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry . 2019;53:742–759. doi: 10.1177/0004867419835028. [DOI] [PubMed] [Google Scholar]
- 101.Jeppesen R, Christensen RHB, Pedersen EMJ, Nordentoft M, Hjorthøj C, Köhler-Forsberg O, Benros ME. Efficacy and safety of anti-inflammatory agents in treatment of psychotic disorders - A comprehensive systematic review and meta-analysis. Brain Behav Immun . 2020;90:364–380. doi: 10.1016/j.bbi.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 102.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol . 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 103.Kuwahara A, Matsuda K, Kuwahara Y, Asano S, Inui T, Marunaka Y. Microbiota-gut-brain axis: enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed Res . 2020;41:199–216. doi: 10.2220/biomedres.41.199. [DOI] [PubMed] [Google Scholar]
- 104.Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet . 2017;174:651–660. doi: 10.1002/ajmg.b.32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe . 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark Insights . 2015;10:47–54. doi: 10.4137/BMI.S22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greenwood J, Acharya RB, Marcellus V, Rey JA. Lumateperone: A Novel Antipsychotic for Schizophrenia. Ann Pharmacother . 2021;55:98–104. doi: 10.1177/1060028020936597. [DOI] [PubMed] [Google Scholar]
- 108.Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother . 2016;16:601–614. doi: 10.1080/14737175.2016.1174577. [DOI] [PubMed] [Google Scholar]
- 109.Edinoff A, Wu N, deBoisblanc C, Feltner CO, Norder M, Tzoneva V, Kaye AM, Cornett EM, Kaye AD, Viswanath O, Urits I. Lumateperone for the Treatment of Schizophrenia. Psychopharmacol Bull . 2020;50:32–59. [PMC free article] [PubMed] [Google Scholar]
- 110.Correll CU, Vanover KE, Davis RE, Chen R, Satlin A, Mates S. Safety and tolerability of lumateperone 42 mg: An open-label antipsychotic switch study in outpatients with stable schizophrenia. Schizophr Res . 2021;228:198–205. doi: 10.1016/j.schres.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 111.Goff DC. Promising Evidence of Antipsychotic Efficacy without Dopamine D2-Receptor Binding. N Engl J Med . 2020;382:1555–1556. doi: 10.1056/NEJMe2001508. [DOI] [PubMed] [Google Scholar]
- 112.Krogmann A, Peters L, von Hardenberg L, Bödeker K, Nöhles VB, Correll CU. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr . 2019;24:38–69. doi: 10.1017/S109285291900124X. [DOI] [PubMed] [Google Scholar]
- 113.Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, Goldman R, Loebel A. A Non-D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N Engl J Med . 2020;382:1497–1506. doi: 10.1056/NEJMoa1911772. [DOI] [PubMed] [Google Scholar]
- 114.Fowler S, Kletzl H, Finel M, Manevski N, Schmid P, Tuerck D, Norcross RD, Hoener MC, Spleiss O, Iglesias VA. A UGT2B10 splicing polymorphism common in african populations may greatly increase drug exposure. J Pharmacol Exp Ther . 2015;352:358–367. doi: 10.1124/jpet.114.220194. [DOI] [PubMed] [Google Scholar]
- 115.Gibert-Rahola J, Villena-Rodriguez A. Glutamatergic drugs for schizophrenia treatment. Actas Esp Psiquiatr . 2014;42:234–241. [PubMed] [Google Scholar]
- 116.Stauffer VL, Millen BA, Andersen S, Kinon BJ, Lagrandeur L, Lindenmayer JP, Gomez JC. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res . 2013;150:434–441. doi: 10.1016/j.schres.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 117.Marek GJ. When is a Proof-of-Concept (POC) not a POC? Curr Pharm Des . 2015;21:3788–3796. doi: 10.2174/1381612821666150605105632. [DOI] [PubMed] [Google Scholar]
- 118.Adams DH, Kinon BJ, Baygani S, Millen BA, Velona I, Kollack-Walker S, Walling DP. A long-term, phase 2, multicenter, randomized, open-label, comparative safety study of pomaglumetad methionil (LY2140023 monohydrate) versus atypical antipsychotic standard of care in patients with schizophrenia. BMC Psychiatry . 2013;13:143. doi: 10.1186/1471-244X-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin CH, Lin CH, Chang YC, Huang YJ, Chen PW, Yang HT, Lane HY. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol Psychiatry . 2018;84:422–432. doi: 10.1016/j.biopsych.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Lane HY, Lin CH, Green MF, Hellemann G, Huang CC, Chen PW, Tun R, Chang YC, Tsai GE. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry . 2013;70:1267–1275. doi: 10.1001/jamapsychiatry.2013.2159. [DOI] [PubMed] [Google Scholar]
- 121.Larsen C, Shahinas J. Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. J Clin Med Res . 2020;12:129–141. doi: 10.14740/jocmr4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Srinivasan S, Tampi RR, Balaram K, Kapoor A. Pimavanserin for the treatment of psychosis in Alzheimer's disease: A literature review. World J Psychiatry . 2020;10:162–174. doi: 10.5498/wjp.v10.i7.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strauss GP, Zamani Esfahlani F, Sayama H, Kirkpatrick B, Opler MG, Saoud JB, Davidson M, Luthringer R. Network Analysis Indicates That Avolition Is the Most Central Domain for the Successful Treatment of Negative Symptoms: Evidence From the Roluperidone Randomized Clinical Trial. Schizophr Bull . 2020;46:964–970. doi: 10.1093/schbul/sbz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al-Nema MY, Gaurav A. Phosphodiesterase as a Target for Cognition Enhancement in Schizophrenia. Curr Top Med Chem . 2020;20:2404–2421. doi: 10.2174/1568026620666200613202641. [DOI] [PubMed] [Google Scholar]
- 125.Macek TA, McCue M, Dong X, Hanson E, Goldsmith P, Affinito J, Mahableshwarkar AR. A phase 2, randomized, placebo-controlled study of the efficacy and safety of TAK-063 in subjects with an acute exacerbation of schizophrenia. Schizophr Res . 2019;204:289–294. doi: 10.1016/j.schres.2018.08.028. [DOI] [PubMed] [Google Scholar]