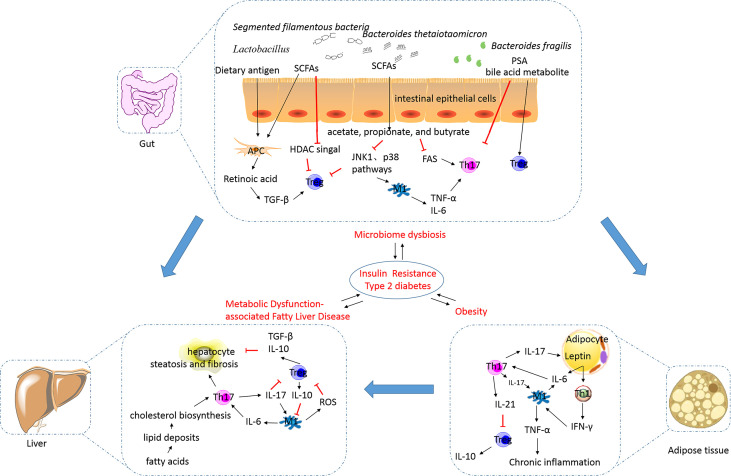
Figure 2.
The alterations in and the roles of the Th17/Treg balance in metabolic disorders. Excess nutrition can result in the development of obesity and metabolic dysfunction-associated fatty liver disease, and this phenomenon is reportedly influenced by the gut microbiota. This results in the development of insulin resistance, diabetes, metabolic syndrome, and related cardiovascular complications (their relationships were showed by blue arrows). In obesity, adipocytes can secrete proinflammatory cytokines, including IL-6, to increase the number of Th17 cells. Th17 cells stimulate macrophages towards an inflammatory signaling cascade. IL-17 secreted by Th17 cells targets adipocytes and participates in the proinflammatory signaling. IL-21 secreted by Th17 cells can inhibit Treg cell differentiation and function. The decreased number of Treg cells in visceral adipose tissues is inversely correlated with the body mass index and plasma leptin levels. Increased release of free fatty acids by white adipose tissue causes hepatocyte injury and leads to the synthesis of proinflammatory cytokines. IL-17A increases cytokine production in hepatocytes and macrophages, thereby inducing steatosis and fibrosis. M1 macrophages can inhibit the function of Treg cells via ROS-induced apoptosis. TGF-β and IL-10 secreted by Treg cells are involved in the exertion of anti-inflammatory effects. The intestinal microbiota is essential for the development of obesity and plays an important role in regulating NAFLD progression. Translocation of bacteria and bacterial products activates inflammasomes and stimulates proinflammatory cytokines to cause a shift in the Th17/Treg balance. Lactobacillus reuteri, Bacteroides fragilis, Bacteroides thetaiotaomicron, Clostridium, and Faecalibacterium prausnitzii promote Treg cell differentiation. Segmented filamentous bacteria are necessary for the development of the gut Th17 cells. SCFAs, as metabolites of microbes such as acetate-, propionate-, and butyrate-producing microbes, can limit Th17 cell and promote Treg cell differentiation. Bile acid transformation mediated by the gut bacteria can increase Foxp3 expression in Treg cells. Th17, T helper 17; Treg, regulatory T; IL, interleukin; TGF, transforming growth factor; SCFA, short-chain fatty acid; PSA, polysaccharide A; HDAC, histone deacetylase; JNK, c-Jun N-terminal kinase; FAS, fatty acid synthesis; TNF-α, tumor necrosis factor alpha; IR, insulin resistance; T2DM, type 2 diabetes mellitus; NAFLD, non-alcoholic fatty liver disease; ROS, reactive oxygen species; IFN-γ, interferon gamma.