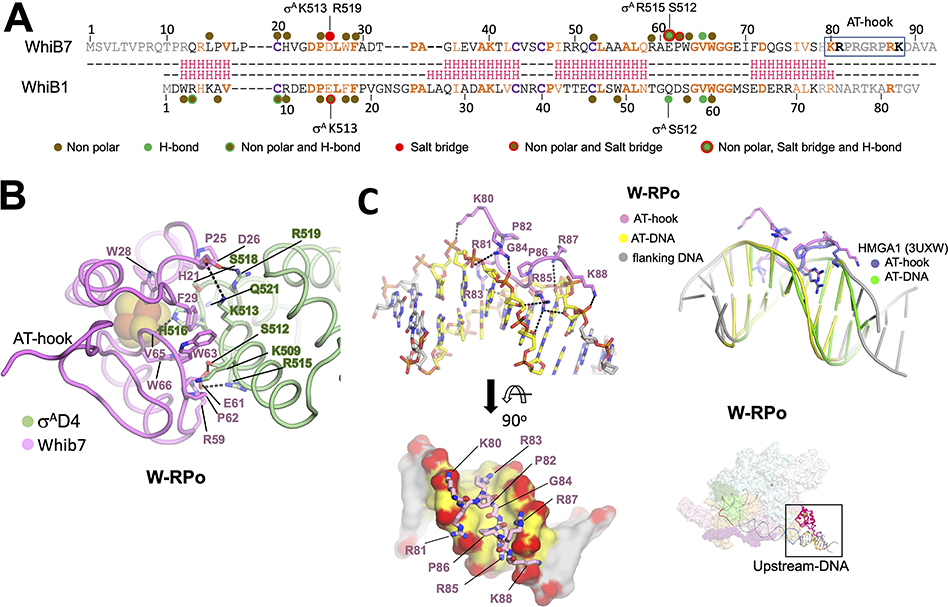
Figure 4. WhiB7 interactions with σAD4 and DNA.
A. Structure-based sequence alignment of Mtb WhiB7 and WhiB1. Residues of WhiB1 that interact with σAD4 (Wan et al., 2020) are highlighted by the nature of interactions and compared to those of WhiB7 (this study). Residues of σAD4 participating in ionic interactions are indicated above or below the sequences. The DNA binding AT-hook motif of WhiB7 is boxed. The interactions between WhiB7 and σAD4 were determined using a 4.5 Å distance cutoff (CCP4) (Winn et al., 2011) between carbon atoms for nonpolar interactions, a 4.5 Å cutoff between basic and acidic side chains for ionic interactions, and a 3.5 Å cutoff for hydrogen bonds between donors and acceptors. Identical residues are bolded orange, homologous residues colored orange, and the conserved cysteines that chelate the 4Fe-4S cluster are in bolded purple. The secondary structure of each protein is shown in red: H indicates helices.
B. Residues of WhiB7 and σAD4 that interact with each other are shown in stick, and each protein’s backbone is shown in cartoon tube. Dotted lines connect residues of σAD4 and WhiB7 that participate in ionic or hydrogen bond interactions. The 4Fe-4S cluster is drawn as spheres.
C. Top left: WhiB7 AT-hook interactions with the AT-rich DNA are indicated. Both the AT-hook and the DNA are drawn as cartoon sticks and colored, as indicated. Bottom left: A top view showing the WhiB7 AT-hook fits intimately into the minor groove of the AT-rich motif. The DNA is rendered in surface, and backbone oxygens are colored red to highlight the ionic and hydrogen bonds between WhiB7 and the DNA. Top Right: an alignment of the AT-rich DNA of W-RPo to that of the crystal structure of the human HMGA1 AT-hook with DNA (Fonfría-Subirós et al., 2012) shows that the AT-hooks superimpose well. Bottom Right: Image in the top inset shows the W-RPo structure for reference with the region of interest boxed.