Table 1.
Summary of the redesign of the complete S glycoprotein used in vaccines against SARS-CoV-2.
| Developers | Vaccine | Vaccine platform | Administration (# doses) | Unfolded S protein design |
|---|---|---|---|---|
| Vaxart | VXA-CoV2-1 | nrVV | Oral (2) |
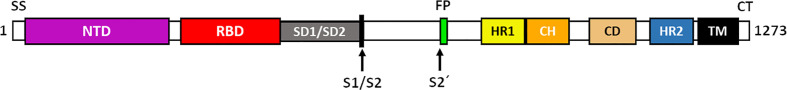
|
| Arcturus Therapeutics | LUNAR-COV-19/ARCT-021 | RNA | ND | |
| AstraZeneca + University of Oxford | Chadox1/AZD1222 | nrVV | IM (2) |
|
| CanSino Biological Inc. + Beijing Institute of Biotechnology | Ad5-nCoV | nrVV | IM (1) | |
| Genexine Consortium | GX-19 | DNA | IM (2) |
|
| ReiThera + Leukocare + Univercells | GRAd-COV2 | nrVV | IM (1) |
|
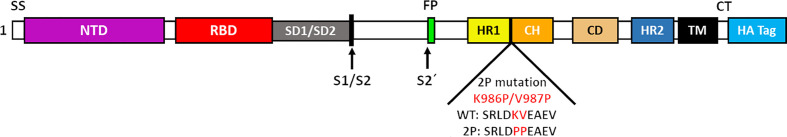
| University of Munich (Ludwig-Maximilians) | MVA-SARS-2-S | nrVV | IM (2) |
|
| Pfizer-Biontech | BNT162b2 | nrVV | IM(2) | |
| Moderna | mRNA-1273 | RNA | IM(2) |
|
| Inovio Pharmaceuticals + International Vaccine Institute + Advaccine (Suzhou) Biopharmaceutical Co., Ltd | INO-4800 | DNA | ID (2) |
|
| Janssen Pharmaceutical | Ad26.COV2.S | nrVV | IM (2) |
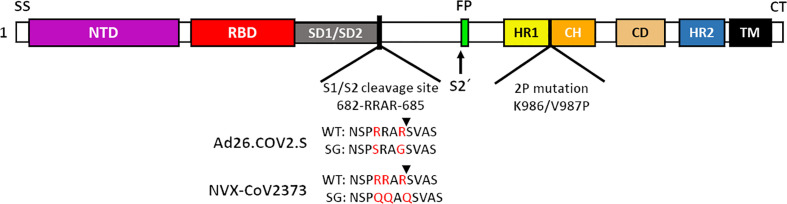
|
| Novavax | NVX-CoV2373 | PS | IM (2) | |
| Clover Biopharmaceuticals Inc. + GSK + Dynavax | SCB-2019 | PS | IM (2) |
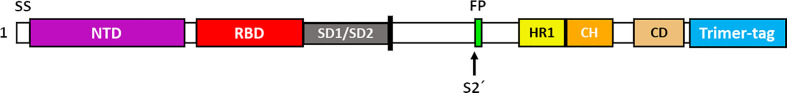
|
| CSL Ltd. + Seqirus + University of Queensland | Sclamp | PS | IM (2) |
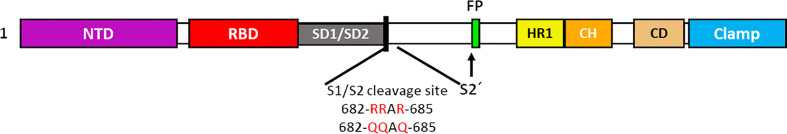
|
| Medicago Inc. | Plant-based VLP / Co-VLP | VLP | IM (2) |
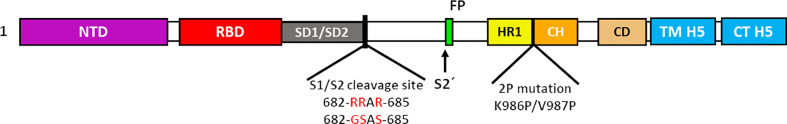
|
| The University of Texas | HexaPro | PS | ND |
|
| Stanford University | SΔC-Fer | VLP | ND |
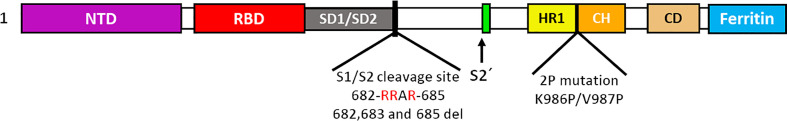
|
Vaccine platform acronym: nrW, non replicating viral vector; RNA, ribonucleic acid; DNA, desoxyribonucleic acid; PS, protein subunit; VLP, virus like particle. Route of administration. Oral, ND, no data; IM, intramuscular; ID, intradermal.
