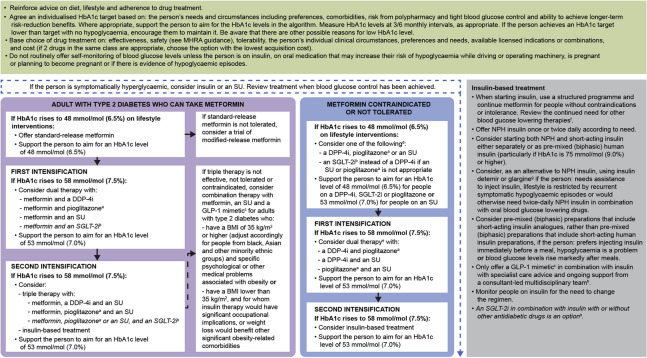
Fig. 4.
NICE treatment algorithm for blood glucose-lowering therapy in adults with T2D. ©NICE [2015] Type 2 diabetes in adults: management NICE guideline [NG28]. [41]. Available from www.nice.org.uk/guidance/ng28. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights) NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication. Recommendations that cover DPP4is, GLP-1 mimetics and SUs refer to these groups of drugs at a class level. aWhen prescribing pioglitazone, exercise particular caution if the person is at high risk of the AEs of the drug. Pioglitazone is associated with increased risk of heart failure, bladder cancer and bone fracture. Known risk factors for these conditions, including increased age, should be carefully evaluated before treatment: see the manufacturers’ summaries of product characteristics for details. MHRA guidance (2011) advises that ‘prescribers should review the safety and efficacy of pioglitazone in individuals after 3–6 months of treatment to ensure that only those deriving benefit continue to be treated’. bSee NICE Technology Appraisal Guidance 288 and 418, 315 and 336 on dapagliflozin, canagliflozin and empagliflozin, respectively. All these SGLT2is are recommended as options in dual therapy regimens with metformin under certain conditions, as options in triple therapy regimens and in combination with insulin. All three are also options as monotherapies in adults in whom metformin is contradicted or not tolerated. Serious and life-threatening cases of DKA have been reported in people taking SGLT2is (canagliflozin, dapagliflozin or empagliflozin) or shortly after stopping the SGLT2i. MHRA guidance (2015) advises testing for raised ketones in people with DKA symptoms, even if plasma glucose levels are near normal. cOnly continue GLP-1 mimetic therapy if the person has a beneficial metabolic response (reduction of HbA1c by at least 1.0% [11 mmol/mol] and weight loss of at least 3% of initial body weight in 6 months). dIf metformin is contradicted or not tolerated, repaglinide is both clinically effective and cost effective in adults with T2D. However, discuss with any person for whom repaglinide is being considered that there is no licensed non-metformin-based combination containing repaglinide that can be offered at first intensification. eDrugs in dual therapy should be introduced in a stepwise manner, checking tolerability and effectiveness of each drug. fMHRA guidance (2011) notes that cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in individuals with risk factors for development of cardiac failure. Patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac status occurs. gThe recommendations in this guideline also apply to any current and future biosimilar product(s) of insulin glargine that have an appropriate marketing authorisation that allows use of the biosimilar(s) in the same indication. AE adverse event, BMI body mass index, DKA diabetic ketoacidosis, DPP4i dipeptidyl peptidase 4 inhibitor, GLP-1 glucagon-like peptide 1, HbA1c glycated haemoglobin, MHRA Medicines and Healthcare Products Regulatory Agency, NICE National Institute for Health and Care Excellence, NPH Neutral Protamine Hagedorn, SGLT2i sodium-glucose cotransporter 2 inhibitor, SU sulfonylurea, T2D type 2 diabetes