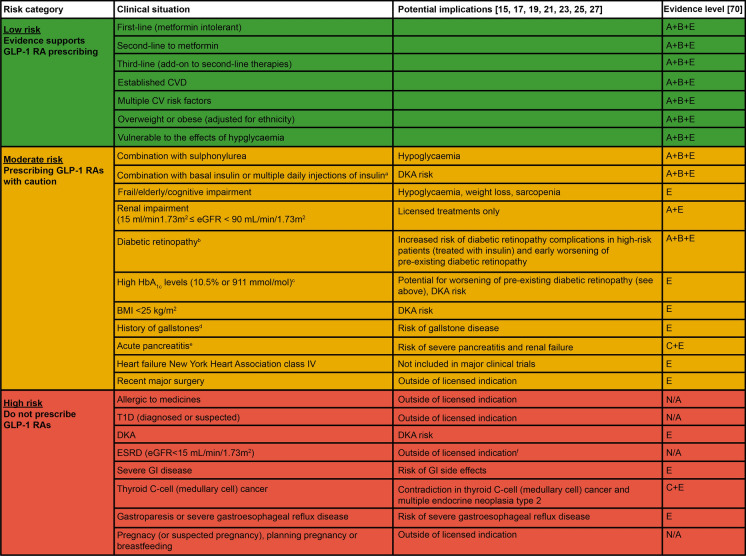
Fig. 7.
Benefit/risk tool: a reference guide regarding the use of GLP-1 RAs in patients with T2D. aGLP-1 RA therapies should be prescribed with caution in people requiring rapid reduction in insulin dose or discontinuation of insulin, because of increased risk of DKA [76]; bGLP-1 RA therapies should be prescribed with caution in people with diabetic retinopathy, because of increased risk of diabetic retinopathy complications in high-risk people (treated with insulin) and early worsening of pre-existing diabetic retinopathy, evidenced in the SUSTAIN 6 study (semaglutide OW) [37]; cHbA1c levels should be monitored regularly and stop GLP-1 RA if elevated levels continue, following treatment initiation; dGLP-1 RAs should be prescribed with caution in people with gallstones, because of increased risk of gallstone diseases, evidenced in the LEADER study (liraglutide OD) [56]; eGLP-1 RAs should be prescribed with caution in people with acute pancreatitis, because of risk of severe pancreatitis and renal failure [16, 18, 20, 22, 24, 26, 28, 77], and exenatide BID is advised to be discontinued by MHRA if pancreatitis is diagnosed [77]; fTo our knowledge, GLP-1 RAs are not recommended in patients with ESRD in European summary of product characteristics; however, there is no eGFR limitation for the use of GLP-1 RAs in some countries (e.g. for semaglutide in the USA). BID twice daily, BMI body mass index, CV cardiovascular, CVD cardiovascular disease, DKA diabetic ketoacidosis, eGFR estimated glomerular filtration rate, ESRD end-stage renal disease, GI gastrointestinal, GLP-1 RA glucagon-like peptide 1 receptor agonist, HbA1c glycated haemoglobin, MHRA Medicines and Healthcare Products Regulatory Agency, N/A not applicable, OD once daily, OW once weekly, SU sulfonylurea, T1D type 1 diabetes, T2D type 2 diabetes