Abstract
Background:
Up to two-thirds of patients with obstructive coronary artery disease (CAD) have silent ischemia (SI), which predicts an adverse prognosis and can be a treatment target in obstructive CAD. Over 50% of women with ischemia and no obstructive CAD have coronary microvascular dysfunction (CMD), which is associated with adverse cardiovascular outcomes. We aimed to investigate the prevalence of SI in CMD in order to consider it as a potential treatment target.
Methods:
36 women with CMD by coronary reactivity testing and 16 age matched reference subjects underwent 24-hr 12-lead ambulatory ECG monitoring (Mortara Instruments) after anti-ischemia medication withdrawal. Ambulatory ECG recordings were reviewed by two-physician consensus masked to subject status for SI measured by evidence of ≥ 1 minute horizontal or downsloping ST segment depression ≥ 1.0 mm, measured 80 ms from the J point.
Results:
Demographics, resting heart rate, and systolic blood pressure were similar between CMD and reference subjects. Thirty-nine percent of CMD women had a total of 26 SI episodes vs. 0 episodes in the reference group (p=0.002). Among these women 13/14 (93%) had SI, and few episodes (3/26, 12%) were symptomatic. Mean HR at the onset of SI was 96±13 bpm and increased to 117±16 bpm during the ischemic episodes. 87% reported symptoms that were not associated with ST depressions.
Conclusions:
Ambulatory ischemia is prevalent in women with CMD, with a majority being SI, while most reported symptoms were not accompanied by ambulatory ischemia. Clinical trials evaluating anti-ischemic medications should be considered in the CMD population.
Keywords: coronary vascular dysfunction, silent ischemia, ambulatory monitoring
Background:
Nearly half of women who undergo coronary angiography for symptoms of myocardial ischemia have no obstructive coronary artery disease (CAD) defined as ≥ 50% stenosis of an epicardial coronary artery. 1, 2 Patients with evidence of ischemia and no obstructive coronary artery disease (INOCA) are increasingly recognized with an estimated prevalence of 3–4 million in United States.3 Current evidence identifies these women at significant risk for adverse cardiovascular events.1, 2, 4–6 Coronary microvascular dysfunction (CMD), from endothelium dependent or independent mechanisms, leads to abnormal myocardial blood flow regulation and may contribute to ischemia in this population.3, 5, 7, 8 CMD is highly prevalent in patients with INOCA, and may be a treatment target, but therapeutic strategies are not well established.
Myocardial ischemia can present with angina or angina equivalents; however, ischemia can also occur in the absence of symptoms, known as silent ischemia (SI). SI is prevalent in as many as two-thirds of obstructive CAD patients, often at relatively low heart rates of 90–120 beats per minute.9, 10 SI on ambulatory ECG monitoring is associated with adverse cardiovascular outcomes including death,10–12 and anti-ischemic therapy directed at SI improves outcomes in obstructive CAD subjects.13, 14 However, the relationship between CMD and SI has not been well characterized in contemporary, well-characterized subjects.15, 16 Given that treatment of CMD to reduce event rates is not established, we aimed to investigate the prevalence of SI in CMD in order to consider treatment of SI with anti-ischemic therapy as a potential treatment target for CMD.
Methods:
Study Subjects
Subjects were recruited from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) Study (PI: Bairey Merz), which aims to study the pathophysiology of women with signs and symptoms of ischemia but no obstructive CAD on angiography.17, 18 As part of the WISE-CVD study, subjects underwent invasive coronary reactivity testing (CRT) to diagnose CMD as previously published.5, 8, 19 Women with CMD from the WISE-CVD study were retrospectively enrolled in the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) sub-study (n=36) if they agreed to further testing in the CANS study. CMD subjects were compared to age matched asymptomatic reference subjects (n=16), recruited for WISE-CVD cardiac magnetic resonance imaging purposes.8 Inclusion and exclusion criteria for CANS were identical to WISE-CVD, which has been previously published.5, 8, 17, 20 In addition to baseline characteristics, clinical, and medication history, the Seattle Angina Questionnaire (SAQ) was collected.21 The reference subjects were asymptomatic individuals with no cardiac risk factors, not on any cardiac medications, and who had a normal exercise treadmill testing. These WISE-CVD and CANS projects were approved by the Institutional Review Board at Cedars-Sinai Medical Center.
Coronary Reactivity Testing Protocol
Coronary reactivity testing (CRT) protocol has been previously published.19 In brief, coronary functional testing was done using intracoronary adenosine, acetylcholine, and nitroglycerin.19 After placement of a Doppler wire (FloWire®, Volcano Corp), in the left anterior descending coronary artery, coronary flow reserve (CFR) to adenosine (18 mcg and 36 mcg) was measured as the ratio of the hyperemic average peak velocity to baseline average peak velocity, and CFR ≤ 2.5 was considered abnormal. Graded intracoronary infusions of acetylcholine (0.364 mcg and 36.4 mcg) were used to asses coronary artery diameter change, and ≤ 5% dilation measured by quantitative coronary angiography was considered abnormal. Coronary blood flow change in response to acetylcholine was calculated as previously published, and change ≤ 50% was abnormal.19 Smooth muscle function was assessed using intracoronary nitroglycerin (200 mcg), with change < 20% was abnormal.
Assessment of Ambulatory ECG Recordings
Participants in the study were monitored by 12-lead 24-hr ambulatory ECG (AECG) (Mortara Instruments®) and were instructed to keep a journal of chest pain or chest pain equivalents while monitored. Medications were held for 24 hours prior to AECG monitoring and during the monitoring period. Long acting calcium channel blockers and nitrates were withheld for 48 hours prior to start of AECG. A trained and experienced technician processed the AECG recordings. A recording was considered eligible if the following criteria were met: over 12 hours of analyzable data, both daytime and nighttime periods available, and sinus rhythm.20 Tracings were reviewed by two physicians masked to clinical information, for evidence of ≥ 1 minute horizontal or down sloping ST segment depression ≥ 1.0 mm, measured 80 ms from the J point. During the 24-hr monitoring subjects were asked to note the time of onset and duration, and describe the symptoms in a diary log. Subject journals were then evaluated to see if symptoms of angina occurred at or near the time of each episode.
Statistical Analysis
Case control analysis was performed using Fisher’s exact test and the Kruskal-Wallis test for categorical and quantitative variables, respectively. Data is presented as n (%) or mean ± SD. Statistically significant values are considered when p ≤ 0.05.
Results
Table 1 compares the women with CMD (n=36) with age matched reference subjects (n=16). Overall, no significant hemodynamic differences were noted other than those with CMD were found to have lower diastolic blood pressures than reference subjects. Over a third of CMD subjects had hypertension and hyperlipidemia, with approximately 10% with diabetes. CMD subjects were more likely to be on aspirin, statins, nitrates, ACE-I, calcium channel blockers and beta blockers than reference subjects as expected due to study design. The mean time between CRT and AECG monitoring was 2.6 ± 1.9 years.
Table 1:
Comparison of CMD and Reference Subjects
| Characteristic | CMD Subjects (n=36) | Reference Subjects (n=16) | p-Value |
|---|---|---|---|
| Age | 57± 10 | 51± 20 | 0.8 |
| Body Mass Index | 27± 6 | 26 ± 4 | 0.7 |
| Systolic Blood Pressure (mmHg) | 118 ± 15 | 122 ± 16 | 0.3 |
| Diastolic Blood Pressure (mmHg) | 62 ± 8 | 68 ± 8 | 0.02 |
| Heart Rate (bpm) | 68 ± 12 | 65 ± 12 | 0.5 |
| Hypertension | 13 (36%) | 0 (0%) | 0.005 |
| Diabetes Mellitus | 4 (11%) | 0 (0%) | 0.3 |
| Hyperlipidemia | 14 (39%) | 0 (0%) | 0.002 |
| Prior Tobacco Use | 9 (25%) | 0 (0%) | 0.04 |
| Statin | 24 (67%) | 0 (0%) | <0.001 |
| Angiotensin Converting Enzyme Inhibitor (ACE-I) | 9 (25%) | 0 (0%) | 0.05 |
| Angiotensin Receptor Blocker | 5 (14%) | 0 (0%) | 0.3 |
| Aspirin | 27 (75%) | 1 (7%) | <0.001 |
| Beta Blocker | 15 (42%) | 0 (0%) | 0.004 |
| Calcium Channel Blocker | 10 (28%) | 0 (0%) | 0.05 |
| Nitrate | 21 (58%) | 0 (0%) | <0.001 |
| Ranolazine | 8 (22%) | 0 (0%) | 0.2 |
| Hormone Therapy | 8 (22%) | 3 (19%) | >0.9 |
| Ambulatory Ischemia* | 14 (39%) | 0 (0%) | 0.002 |
| Silent Ischemia^ | 13 (36%) | 0 (0%) | 0.005 |
Defined as evidence of ≥ 1 minute horizontal or downsloping ST segment depression ≥ 1.0 mm, measured 80 ms from the J point on ambulatory monitoring; CMD = coronary microvascular dysfunction
Among those with ambulatory ischemia, the absence of chest pain or chest pain equivalents.
Silent Ischemia on Ambulatory Monitoring
In the CMD group, 39% (14/36) had at least one episode of ST depression on ambulatory monitoring compared to 0 subjects in the reference group (p=0.002). Among CMD subjects, we compared those with ambulatory ischemia to those without ambulatory ischemic ST depressions, and found no differences in clinical characteristics (Table 2). There were a total of 26 episodes of ST depressions in 14 CMD subjects. Only three (12%) of these episodes of ST depressions were symptomatic when time periods of ischemic ST depressions were compared to angina or angina-equivalents reported in subject diaries. A total of 23 SI episodes were recorded in 13 CMD subjects (Figure). One CMD subject had 4 episodes of chest pain, and only one was associated with ST depressions. Another CMD subject had 4 episodes of ST depressions, of which 2 were symptomatic and 2 were SI. Three out of 23 SI episodes (13%) were during nighttime (10 PM to 6 AM). The distribution of SI episodes largely favored inferior leads (II, III, or aVF) with 87% of the episodes involving these leads. The mean heart rate (HR) at onset of SI was found to be 96±13 bpm and the mean peak HR was 117±16 bpm. The mean difference of HR change from prior to SI episode to peak HR during SI was 21±15 bpm. 61% of SI episodes occurred at HR < 100 bpm prior to the onset of SI episode. There was a trend of more SI episodes at peak HR >120 bpm compared to ≤ 120 bpm (p=0.06). There were no differences in the number of ischemic episodes that occurred with a heart rate change of ≥20 bpm or <20 bpm. A majority of episodes occurred with a change of HR less than 30 bpm from baseline.
Table 2:
Comparison of CMD subjects Stratified by Ambulatory Ischemia
| Characteristics | Ambulatory ST Depressions (14)** | No ST Depressions (22) | p-value |
|---|---|---|---|
| Age (years) | 59±7 | 56±11 | 0.3 |
| Body Mass Index | 27±5 | 27±6 | 0.9 |
| Systolic Blood Pressure (mmHg) | 119±16 | 118±15 | 0.9 |
| Diastolic Blood Pressure (mmHg) | 61±8 | 62±8 | 0.6 |
| Heart Rate (bpm) | 63±8 | 70±13 | 0.2 |
| Hypertension | 4 (29%) | 9 (41%) | 0.5 |
| Diabetes Mellitus | 1 (7%) | 3 (14%) | >0.9 |
| Hyperlipidemia | 5 (36%) | 9 (41%) | >0.9 |
| Tobacco Use | 6 (43%) | 3 (14%) | 0.1 |
| Statin | 11 (79%) | 13 (59%) | 0.3 |
| ACE-I | 6 (43%) | 3 (14%) | 0.1 |
| Angiotensin Receptor Blocker | 1 (7%) | 4 (18%) | 0.6 |
| Aspirin | 10 (71%) | 17 (77%) | 0.7 |
| Beta Blocker | 6 (43%) | 9 (41%) | >0.9 |
| Calcium Channel Blocker | 2 (14%) | 8 (36%) | 0.3 |
| Nitrate | 10 (71%) | 11 (50%) | 0.3 |
| Ranolazine | 1 (7%) | 7 (32%) | 0.1 |
| Hormone Therapy | 4 (29%) | 4 (18%) | 0.7 |
Includes SI and non-SI subjects.
CMD = coronary microvascular dysfunction
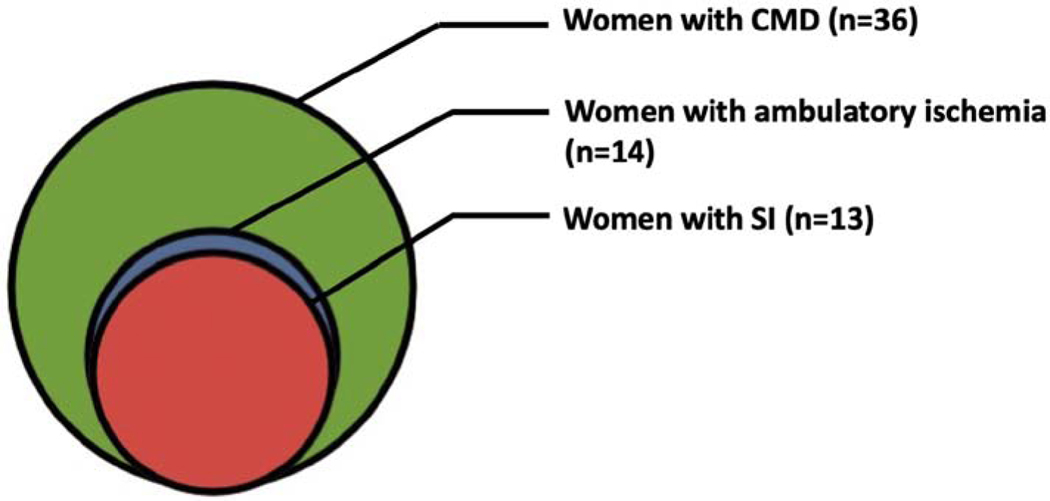
Figure :
Silent ischemia is highly prevalent in women with coronary microvascular dysfunction despite having no obstructive epicardial coronary artery stenosis. CMD = Coronary microvascular dysfunction, SI = Silent ischemia
CRT Results and Ambulatory Ischemia
There were no differences in CRT measures comparing those with ambulatory ischemia (n=14) vs. those without ambulatory ischemia (n=22): (a) coronary flow reserve to adenosine: 2.88±0.97 vs. 2.67±0.63 (p=0.9); (b) % Δ coronary diameter to acetylcholine: −6.9±21.4 vs. −1.4±18.8 (p=0.3); (c) % Δ coronary blood flow to acetylcholine: 72.2±103.5 vs. 81.0±81.9 (p=0.5); (d) %Δ coronary diameter to nitroglycerin: 18.4±10.6 vs. 11.6±15.5 (p=0.1). There were also no differences in CRT measures between SI vs. non-SI group. Those with ≥ 2 CRT abnormalities (n=11) did not have more SI episodes compared to those with <2 abnormal CRT pathways (n=3) (p= 0.30).
Symptom Burden
Among the CMD women, 15 out of 36 (42%) reported angina symptoms such as chest pressure, dyspnea, or lightheadedness in their symptom diaries during 24-hour monitoring. Among those who reported angina, 13 (87%) reported symptoms that were not associated with ST depressions during the 24 hours of ambulatory monitoring. CMD subjects had a high symptom burden as determined by the five subscales of the SAQ (mean ± SD): (a) physical limitation: 75.0±20.7; (b) angina stability: 56.9±24.4; (c) angina frequency: 62.5±26.4; (d) treatment satisfaction: 87.3±15.3; and (e) quality of life: 64.8±23.6. There was no difference in the SAQ scores between those with and without ambulatory ischemia in all five SAQ domains. There were no differences in SAQ measures between SI vs. non-SI group.
Discussion
To our knowledge, this is the first report on prevalence of SI in a population of women who have undergone CRT to diagnose CMD. We demonstrate that ambulatory ischemia detected by ST segment depressions on 24-hour AECG monitoring is prevalent in over one-third of women with CMD compared to matched reference subjects. Furthermore, a majority of these ST depressions are SI and occurred during the daytime. CMD women in this study demonstrated a high burden of angina, consistent with prior reports.22, 23 While over a third of CMD subjects reported symptoms during 24-hour period, their symptom experience was not associated with ST segment depressions in a majority of cases. Specific CRT measures were not different among those with vs. without SI.
We have previously documented objective evidence of myocardial ischemia in a similar cohort of patients, by phosphorus cardiac magnetic resonance spectroscopy.24 We have also documented an almost 8% myocardial scar in CMD population, detected by cardiac MRI late gadolinium enhancement (LGE), and approximately 1% annual new LGE in women with CMD, the majority of whom do not have a clinical history of myocardial infarction.25 Our finding of a high prevalence of ambulatory ischemia and specifically SI extends these findings and support further investigation into understanding mechanistic pathways in order to develop treatment targets. Recurrent episodes of ischemia have been demonstrated via biopsy to be associated with anatomic changes including myocardial cellular degeneration, increased fibrosis, and hypertrophy.26, 27 These anatomic changes, in addition to the ischemic events, are likely contributing factors to the increased rates of fatal arrhythmias noted in individuals with SI.28
Interestingly, nearly two-thirds of SI episodes in CMD subjects occurred at heart rates less than 100 bpm prior to the onset of ST segment depression. The mean resting HR in the CMD group increased approximately 20 bpm prior to the onset of SI; therefore, it is possible that demand related ischemia may be the explanation of ST segment depression. Our observed heart rate range for SI in CMD is comparable to prior reports in the obstructive CAD and previously termed “cardiac syndrome X” (CSX) populations.9, 29, 30
Historical CSX literature has described prevalent ambulatory ischemia that is dominantly SI and occurs at relatively low heart rates. 31–33 In a study looking at autonomic nervous system dysfunction, 14 (61%) out of 23 patients with CSX had 1 or more episodes of ST depression in 24-hour monitoring. Only one of the 14 subjects had chest pain during the transient ST segment depression.15 CSX described in the literature included heterogeneous groups with chest pain and no obstructive CAD who may or may not have had CMD, because no objective measures of coronary flow reserve or endothelial function were obtained.34 Our results extend prior findings in CSX population to a rigorously defined cohort of women with CMD. Further, our reference control subject comparison confirms these are not false positive ECG findings, and questions prior reports of false positives in population studies not densely phenotyped.
Prior work in obstructive CAD has demonstrated that ischemic episodes on ambulatory monitoring independently predict adverse outcomes.12, 35–37 In 107 patients with chronic stable angina, the 46 (43%) subjects with one or more episode of SI were found to have a threefold increase in risk of cardiac death in the two year follow up period compared to those with no SI (24% vs. 8%, p=0.023).11 However, adverse outcomes from SI has not previously been investigated in CMD subjects diagnosed by comprehensive CRT.
Our current results also demonstrate that the CMD subjects have a high burden of chest pain which appears to be independent of ambulatory ischemia. The mechanisms contributing to cardiac pain are multi-factorial and complex.38 Patients with CMD have heightened nociception, evidenced by a higher pain sensitivity and perception with contrast injection in the catheterization lab, during right ventricular pacing, and during adenosine infusion.39, 40 Cerebral cortical dysfunction and abnormal neural processing has also been suggested in SI.23 In a study of non-diabetic patients with exercise induced ischemia, less cortical activation was found in asymptomatic patients compared to symptomatic patients with angina.23 Rosen et al have reported brain activation in regions such as the prefrontal cortex and the left inferior anterior cingulate during angina in patients with CAD.23, 41 In contrast, in those with silent ischemia there was a failure of frontal cortex activation, although there was thalamic activation (similarly to the angina group).41 They concluded that abnormal CNS processing of afferent pain signals from the heart may be playing a role in SI.23 Chemical substances such as adenosine and dipyridamole mediate chest pain but failed to induce electrographic ischemic changes.10, 42, 43
Mechanical stretching of the coronary arteries is also proposed as a mechanism supported by observations that some individuals experience pain with higher balloon inflation pressure during percutaneous transluminal coronary angioplasty (PTCA).10, 44 In addition to autonomic neuropathy, several biomarkers have also been associated with SI. Beta-endorphins, peripheral benzodiazepine receptors and anti-inflammatory cytokines are increased in patients with SI compared to symptomatic counterparts.22, 45–47 Clearly, more investigation in the brain-heart axis and contributions to angina and SI in CMD population is needed.
While we note a high prevalence of SI in CMD, whether treatment of SI in the CMD population would lead to improved outcomes is yet to be determined. SI has been used as a treatment target in several ischemic heart disease studies, such as the Atenolol Silent Ischemia Study (ASIST) randomized individuals with a history of SI to atenolol vs. placebo.49 The atenolol group demonstrated a significant decrease in primary outcomes including MI, arrhythmias, and death compared to the placebo group,49 which was accompanied by a significant decrease in episodes of SI (p < 0.001).49 In the Asymptomatic Cardiac Ischemia Pilot (ACIP) study, three groups with SI were compared: angina-guided therapies, ischemia-guided therapies, and revascularization.50 A graded reduction in adverse cardiovascular outcomes and episodes of ischemia were observed (0% in revascularization, 1.6% ischemia-guided, and 4.4% angina-guided, p=0.004).50 The Total Ischemic Burden Bisoprolol Study (TIBBS) found that individuals treated to 100% resolution of their ischemic episodes had fewer adverse outcomes as compared to those with residual episodes (17.5% vs. 32.3%, p=0.008).50
Study Limitations.
Our relatively small sample size of cases and reference subjects prevents us from making any conclusions about whether specific endothelium-dependent or independent vasomotor pathways are associated with more SI. Additionally, while medications were withdrawn during ambulatory monitoring, our patients had been treated on anti-ischemic, anti-anginal medications prior to their inclusion in this study, and thus represent a “treated” population which may underestimate SI prevalence. There is also the concern that withdrawal of medications for AECG monitoring may enhance ischemia, which could influence AECG results in our CMD group. We also did not systematically collect information on conditions that impact heart rates and ST segment changes, such as sleep, physical, and mental activity. This study was strengthened by the inclusion of women with objective evidence of CMD diagnosed by CRT in the setting of no obstructive CAD.
Conclusions:
Our results demonstrate that over one-third of women with CMD have SI detected by ambulatory monitoring, while conversely most angina is not related to ambulatory ischemia, both similar to obstructive CAD findings. Given the previously established adverse prognostic significance of SI in obstructive CAD, and the poor outcomes associated with CMD, clinical trials evaluating anti-ischemic medications should be considered in the CMD population. Future studies need to target longer duration of ambulatory monitoring to detect SI burden in women with CMD and its relation to adverse cardiovascular outcomes.
Highlights:
Silent ischemia (SI) is prevalent in women with coronary microvascular dysfunction (CMD) compared to reference controls.
SI occurs in CMD at heart rates similar to obstructive CAD population.
Symptoms of CMD were not accompanied by ambulatory ischemia.
Treatment of SI with anti-ischemic therapy could be a potential treatment for CMD.
Acknowledgments
This work was supported by an unrestricted research grant from Gilead Sciences, and contracts from the National Heart, Lung and Blood Institutes nos. K23HL105787, N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California. MD Nelson was supported by the American Heart Association (16SDG27260115) and the Harry S. Moss Heart Trust. nih.gov identifier: NCT01568177
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ and Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN and Committee ACiW. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66:1918–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bairey Merz CN, Pepine CJ, Walsh MN and Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugiardini R, Manfrini O, Pizzi C, Fontana F and Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–23. [DOI] [PubMed] [Google Scholar]
- 5.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA, National Heart L and Blood I. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:722–5. [DOI] [PubMed] [Google Scholar]
- 6.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ and Ahmed B. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G and Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, Li D, Sharif B, Berman DS, Petersen JW, Pepine CJ and Bairey Merz CN. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panza JA, Quyyumi AA, Diodati JG, Callahan TS and Epstein SE. Prediction of the frequency and duration of ambulatory myocardial ischemia in patients with stable coronary artery disease by determination of the ischemic threshold from exercise testing: importance of the exercise protocol. J Am Coll Cardiol. 1991;17:657–63. [DOI] [PubMed] [Google Scholar]
- 10.Cohn PF, Fox KM and Daly C. Silent myocardial ischemia. Circulation. 2003;108:1263–77. [DOI] [PubMed] [Google Scholar]
- 11.Deedwania PC and Carbajal EV. Silent ischemia during daily life is an independent predictor of mortality in stable angina. Circulation. 1990;81:748–56. [DOI] [PubMed] [Google Scholar]
- 12.Rocco MB, Nabel EG, Campbell S, Goldman L, Barry J, Mead K and Selwyn AP. Prognostic importance of myocardial ischemia detected by ambulatory monitoring in patients with stable coronary artery disease. Circulation. 1988;78:877–84. [DOI] [PubMed] [Google Scholar]
- 13.Stern S, Cohn PF and Pepine CJ. Silent myocardial ischemia. Curr Probl Cardiol. 1993;18:301–59. [DOI] [PubMed] [Google Scholar]
- 14.Pepine CJ. beta-blockers or calcium antagonists in silent ischaemia? Eur Heart J. 1993;14 Suppl F:7–14. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Rosano GM, Amadi AA, Collins P, Coats AJ, Poole-Wilson PA and Kaski JC. Transient autonomic dysfunction precedes ST-segment depression in patients with syndrome X. Am J Cardiol. 1996;77:942–7. [DOI] [PubMed] [Google Scholar]
- 16.Cannon RO, 3rd, Camici PG and Epstein SE. Pathophysiological dilemma of syndrome X. Circulation. 1992;85:883–92. [DOI] [PubMed] [Google Scholar]
- 17.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL and Sopko G. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–61. [DOI] [PubMed] [Google Scholar]
- 18.Pepine CJ, Balaban RS, Bonow RO, Diamond GA, Johnson BD, Johnson PA, Mosca L, Nissen SE, Pohost GM, National Heart L, Blood I and American College of Cardiology F. Women’s Ischemic Syndrome Evaluation: current status and future research directions: report of the National Heart, Lung and Blood Institute workshop: October 2–4, 2002: Section 1: diagnosis of stable ischemia and ischemic heart disease. Circulation. 2004;109:e44–6. [DOI] [PubMed] [Google Scholar]
- 19.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ and Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, Mehta P, Zhang X, Thomson LE, Berman DS, Li D and Bairey Merz CN. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging. 2014;7:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M and Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. [DOI] [PubMed] [Google Scholar]
- 22.Crea F and Gaspardone A. New look to an old symptom: angina pectoris. Circulation. 1997;96:3766–73. [DOI] [PubMed] [Google Scholar]
- 23.Rosen SD, Paulesu E, Nihoyannopoulos P, Tousoulis D, Frackowiak RS, Frith CD, Jones T and Camici PG. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med. 1996;124:939–49. [DOI] [PubMed] [Google Scholar]
- 24.Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, Sharaf BL, Reis S, Kelsey SF and Pohost GM. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. [DOI] [PubMed] [Google Scholar]
- 25.Wei J, Bakir M, Darounian N, Li Q, Landes S, Mehta PK, Shufelt CL, Handberg EM, Kelsey SF, Sopko G, Pepine CJ, Petersen JW, Berman DS, Thomson LEJ and Merz CNB. Myocardial Scar Is Prevalent and Associated With Subclinical Myocardial Dysfunction in Women With Suspected Ischemia But No Obstructive Coronary Artery Disease. Circulation. 2018;137:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaper J. Effects of multiple ischaemic events on human myocardium--an ultrastructural study. Eur Heart J. 1988;9 Suppl A:141–9. [DOI] [PubMed] [Google Scholar]
- 27.Hess OM, Schneider J, Nonogi H, Carroll JD, Schneider K, Turina M and Krayenbuehl HP. Myocardial structure in patients with exercise-induced ischemia. Circulation. 1988;77:967–77. [DOI] [PubMed] [Google Scholar]
- 28.Myerburg RJ, Kessler KM, Mallon SM, Cox MM, deMarchena E, Interian A, Jr. and Castellanos A. Life-threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary-artery spasm. N Engl J Med. 1992;326:1451–5. [DOI] [PubMed] [Google Scholar]
- 29.Wimmer NJ, Scirica BM and Stone PH. The clinical significance of continuous ECG (ambulatory ECG or Holter) monitoring of the ST-segment to evaluate ischemia: a review. Prog Cardiovasc Dis. 2013;56:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deedwania PC and Nelson JR. Pathophysiology of silent myocardial ischemia during daily life. Hemodynamic evaluation by simultaneous electrocardiographic and blood pressure monitoring. Circulation. 1990;82:1296–304. [DOI] [PubMed] [Google Scholar]
- 31.Guzik P, Rogacka D, Trachalski J, Minczykowski A, Balinski M, Wykretowicz A and Wysocki H. Comparison of the exercise treadmill test and 24-hour ECG Holter monitoring in patients with syndrome X or coronary atherosclerosis. Kardiol Pol. 2007;65:262–9; discussion 270–1. [PubMed] [Google Scholar]
- 32.Kaski JC, Crea F, Nihoyannopoulos P, Hackett D and Maseri A. Transient myocardial ischemia during daily life in patients with syndrome X. Am J Cardiol. 1986;58:1242–7. [DOI] [PubMed] [Google Scholar]
- 33.Lanza GA, Manzoli A, Pasceri V, Colonna G, Cianflone D, Crea F and Maseri A. Ischemic-like ST-segment changes during Holter monitoring in patients with angina pectoris and normal coronary arteries but negative exercise testing. Am J Cardiol. 1997;79:1–6. [DOI] [PubMed] [Google Scholar]
- 34.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN and Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 35.Conti CR, Bavry AA and Petersen JW. Silent ischemia: clinical relevance. J Am Coll Cardiol. 2012;59:435–41. [DOI] [PubMed] [Google Scholar]
- 36.Weiner DA, Ryan TJ, McCabe CH, Ng G, Chaitman BR, Sheffield LT, Tristani FE and Fisher LD. Risk of developing an acute myocardial infarction or sudden coronary death in patients with exercise-induced silent myocardial ischemia. A report from the Coronary Artery Surgery Study (CASS) registry. Am J Cardiol. 1988;62:1155–8. [DOI] [PubMed] [Google Scholar]
- 37.Wohlgelernter D, Jaffe CC, Cabin HS, Yeatman LA, Jr. and Cleman M. Silent ischemia during coronary occlusion produced by balloon inflation: relation to regional myocardial dysfunction. J Am Coll Cardiol. 1987;10:491–8. [DOI] [PubMed] [Google Scholar]
- 38.Cannon RO, 3rd. Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol. 2009;54:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagerqvist B, Sylven C and Waldenstrom A. Lower threshold for adenosine-induced chest pain in patients with angina and normal coronary angiograms. Br Heart J. 1992;68:282–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frobert O, Arendt-Nielsen L, Bak P, Funch-Jensen P and Peder Bagger J. Pain perception and brain evoked potentials in patients with angina despite normal coronary angiograms. Heart. 1996;75:436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen SD and Camici PG. The brain-heart axis in the perception of cardiac pain: the elusive link between ischaemia and pain. Ann Med. 2000;32:350–64. [DOI] [PubMed] [Google Scholar]
- 42.Crea F, Pupita G, Galassi AR, el-Tamimi H, Kaski JC, Davies G and Maseri A. Role of adenosine in pathogenesis of anginal pain. Circulation. 1990;81:164–72. [DOI] [PubMed] [Google Scholar]
- 43.Sylven C, Beermann B, Jonzon B and Brandt R. Angina pectoris-like pain provoked by intravenous adenosine in healthy volunteers. British medical journal (Clinical research ed). 1986;293:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomai F, Crea F, Gaspardone A, Versaci F, Esposito C, Chiariello L and Gioffre PA. Mechanisms of cardiac pain during coronary angioplasty. J Am Coll Cardiol. 1993;22:1892–6. [DOI] [PubMed] [Google Scholar]
- 45.Mazzone A, Cusa C, Mazzucchelli I, Vezzoli M, Ottini E, Pacifici R, Zuccaro P and Falcone C. Increased production of inflammatory cytokines in patients with silent myocardial ischemia. J Am Coll Cardiol. 2001;38:1895–901. [DOI] [PubMed] [Google Scholar]
- 46.Mazzone A, Mazzucchelli I, Vezzoli M, Ottini E, Auguadro C, Serio A and Falcone C. Increased expression of peripheral benzodiazepine receptors on leukocytes in silent myocardial ischemia. J Am Coll Cardiol. 2000;36:746–50. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed AH, Shankar K, Eftekhari H, Munir M, Robertson J, Brewer A, Stupin IV and Casscells SW. Silent myocardial ischemia: Current perspectives and future directions. Exp Clin Cardiol. 2007;12:189–96. [PMC free article] [PubMed] [Google Scholar]
- 48.Falcone C, Guasti L, Ochan M, Codega S, Tortorici M, Angoli L, Bergamaschi R and Montemartini C. Beta-endorphins during coronary angioplasty in patients with silent or symptomatic myocardial ischemia. J Am Coll Cardiol. 1993;22:1614–20. [DOI] [PubMed] [Google Scholar]
- 49.Pepine CJ, Cohn PF, Deedwania PC, Gibson RS, Handberg E, Hill JA, Miller E, Marks RG and Thadani U. Effects of treatment on outcome in mildly symptomatic patients with ischemia during daily life. The Atenolol Silent Ischemia Study (ASIST). Circulation. 1994;90:762–8. [DOI] [PubMed] [Google Scholar]
- 50.Rogers WJ, Bourassa MG, Andrews TC, Bertolet BD, Blumenthal RS, Chaitman BR, Forman SA, Geller NL, Goldberg AD, Habib GB and et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study: outcome at 1 year for patients with asymptomatic cardiac ischemia randomized to medical therapy or revascularization. The ACIP Investigators. J Am Coll Cardiol. 1995;26:594–605. [DOI] [PubMed] [Google Scholar]