Figure 3. Clinical utility validation 2: HCC risk after HCV cure by DAA (nested case-control series).
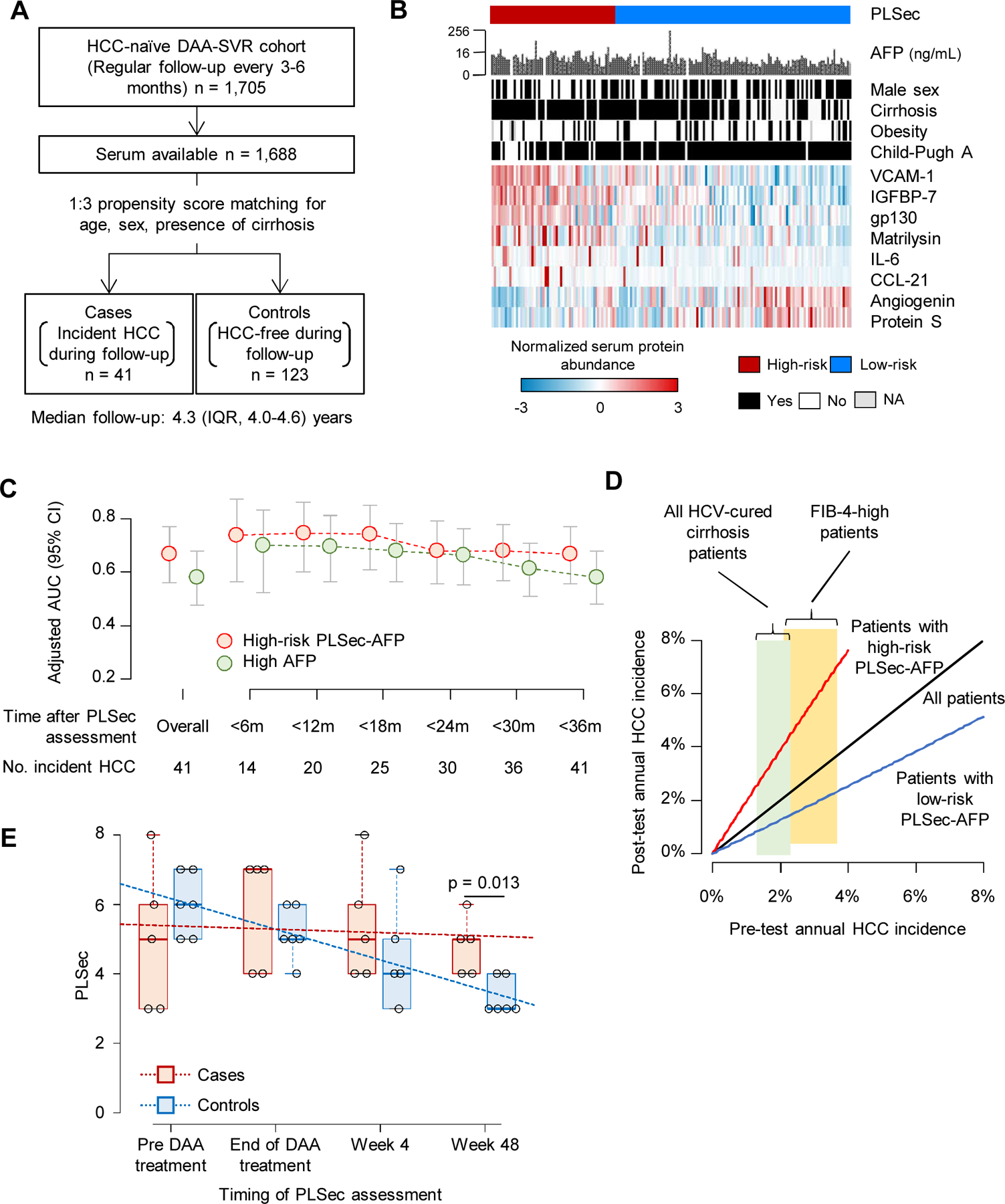
(A) Study design. (B) Patten of the PLSec protein abundance and associated clinical variables. (C) Adjusted AUC of PLSec-AFP score (≥ 1.66) and AFP (≥ 5.5 ng/mL) over time. (D) Pre- and post-test annual HCC incidence rate estimated based on the performance of PLSec-AFP in validation set 2. Widths of light green and yellow boxes indicate ranges of reported annual HCC incidence in all HCV-cured cirrhosis patients and their subset with high FIB-4 index, respectively. (E) Change in PLSec over the course of DAA-based anti-HCV treatment and post-treatment follow-up. Trend of change in PLSec over time was tested by Jonckheere-Terpstra test (p=0.43 for the cases; p <0.001 for the controls). PLSec values at week 48 were lower in the controls compared to the cases (Wilcoxon rank-sum test, p=0.013).
See also Figure S1 and Table S2, 3.
HCC, hepatocellular carcinoma; DAA, direct-acting antivirals; SVR, sustained virologic response; IQR, interquartile range; PLSec, prognostic liver secretome signature; AFP, alpha-fetoprotein; AUC, area under receiver operating characteristic curve; CI, confidence interval; HCV, hepatitis C virus.
