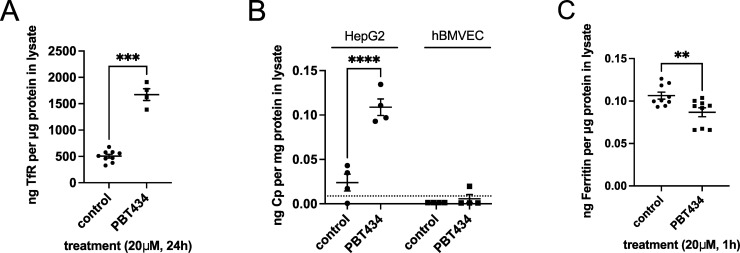
Fig 8. ELISA analysis of iron metabolic protein abundance in PBT434-treated hBMVEC.
(A) hBMVEC were incubated in the absence or presence of 20 μM PBT434 for 24h. Cell lysates were assayed for transferrin receptor (TfR) by sandwich ELISA and normalized to total protein content. Data are represented as mean ± SEM, n = 4–9 biological replicates. Statistical significance was tested using t-test; ****, p < 0.0001, compared to control. The mean values were: 505 ± 109 (control) and 1672 ± 223 ng TfR/μg total cell protein (+PBT434). (B) hBMVEC or HepG2 were grown in monolayers for 24h in media containing serum, then incubated in the absence or presence of 20 μM PBT434 in media without serum for an additional 24h. Following incubation, cells were treated with PI-PLC (0.5 U/ml) for 1h to release any GPI-anchored cell surface proteins. Media was collected, concentrated, assayed for Cp protein by sandwich ELISA and normalized to total protein content. Data are represented as mean ± SEM, n = 4 biological replicates. Statistical significance was tested using t-test; ****, p < 0.0001, compared to control. The lower sensitivity limit of this kit is reported to be 0.12 ng/ml (or 0.0088 ng/mg protein average) indicated by the dashed line. (C) hBMVEC were loaded with 1 μM Fe2+-citrate in RPMI+serum media for 24h, then washed and incubated in the absence or presence of 20 μM PBT434 for an additional 1h. Cell lysates were assayed for ferritin protein by sandwich ELISA and normalized to total protein content. Data are represented as mean ± SEM, n = 9 biological replicates. Statistical significance was tested using t-test; *, p < 0.05, compared to control. The mean values were: 106 ±4 (control) and 87 ± 5 pg Ft/μg total cell protein (+PBT434).