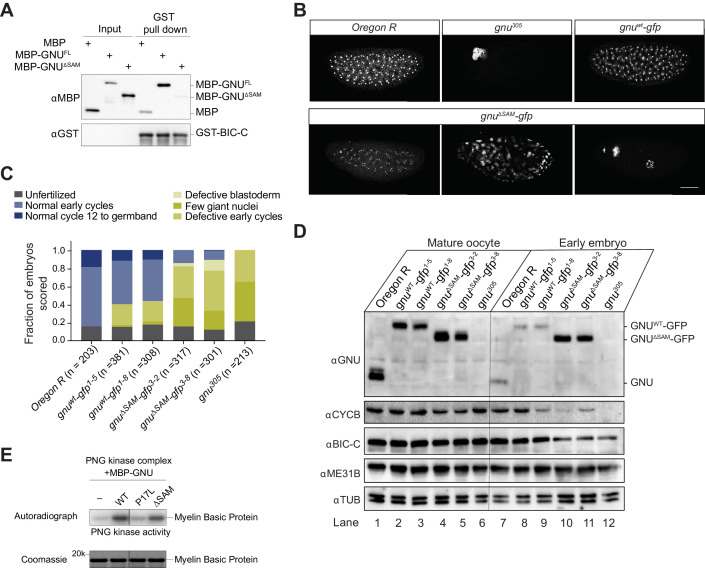
Figure 2. Deletion of the SAM domain of GNU reduces the interaction with BIC-C and confers partial GNU function.
(A) Immunoblot analysis of in vitro pull-down of GNU by BIC-C. Recombinantly expressed and purified MBP-tagged full-length GNU or GNUΔSAM was incubated with GST-BIC-C followed by a GST pull-down. As a control, GST pull-down after incubation with MBP was performed. In contrast to the robust pull-down of MBP-GNU, only slight amounts of MBP-GNUΔSAM were pulled down by GST-BIC-C. The levels of GST-BIC-C pulled down are comparable between all samples. (B, C) Fertilized embryos were collected for 2 hr from wild-type (Oregon R), gnuwt-gfp (gnuwt-gfp; gnu305/ gnu305), gnuΔSAM-gfp (gnuΔSAM-gfp; gnu305/gnu305), or gnu305 (gnu305/gnu305) females. Embryos were fixed and stained with DAPI. (B) Representative images of embryonic phenotypes. The embryos from wild-type and gnuwt-gfp mothers show normal early nuclear division cycles, whereas the gnu305 embryo shows only a few giant nuclei. These nuclei are the consequence of DNA replication in the absence of nuclear division; the number of separate nuclei depends on whether polyploid polar bodies fuse and whether any mitotic divisions occur (Freeman and Glover, 1987; Lee et al., 2003). The gnuΔSAM-gfp embryos show (from left to right panels) normal early cycles, defective blastoderm, and a few giant nuclei. Scale bar represents 100 μm. (C) Quantification of the embryonic phenotypes. Two independent transgenic lines were analyzed for gnuwt-gfp (gnuwt-gfp1-5 and gnuwt-gfp1-8) and gnuΔSAM-gfp (gnuΔSAM-gfp3-2 and gnuΔSAM-gfp3-8). At least 300 embryos were scored for each transgenic line and at least 200 for the Oregon R and gnu305 controls. (D) Immunoblot analysis of protein levels in mature oocytes and embryos from gnu mutants. Extracts were made from mature oocytes and embryos collected for 1 hr from Oregon R, gnuwt-gfp, gnuΔSAM-gfp, and gnu305 females, and the levels of GNU, CYCB, BIC-C, and ME31B were examined by immunoblot. αTUB was used as a loading control. Two independent transgenic gnuwt-gfp and gnuΔSAM-gfp lines were examined. 30 oocytes or embryos were collected for each sample, and the equivalent of 10 oocytes was loaded into the gel per sample. Shown is one of two biological replicates. (E) In vitro assay of PNG kinase activity. Purified MBP-tagged GNUWT, GNUΔSAM, or GNUP17Lwas incubated with the recombinant PNG kinase complex and Myelin Basic Protein (an in vitro phosphorylation target of PNG). Levels of phosphorylation of Myelin Basic Protein by PNG with radiolabeled phosphate were measured by autoradiography. MBP-GNUP17L was used as a negative control, as this amino acid change affects the ability of GNU to activate PNG kinase. In contrast, both GNUWT and GNUΔSAM activate PNG kinase. The levels of Myelin Basic Protein are comparable across samples, as assessed by Coomassie staining.