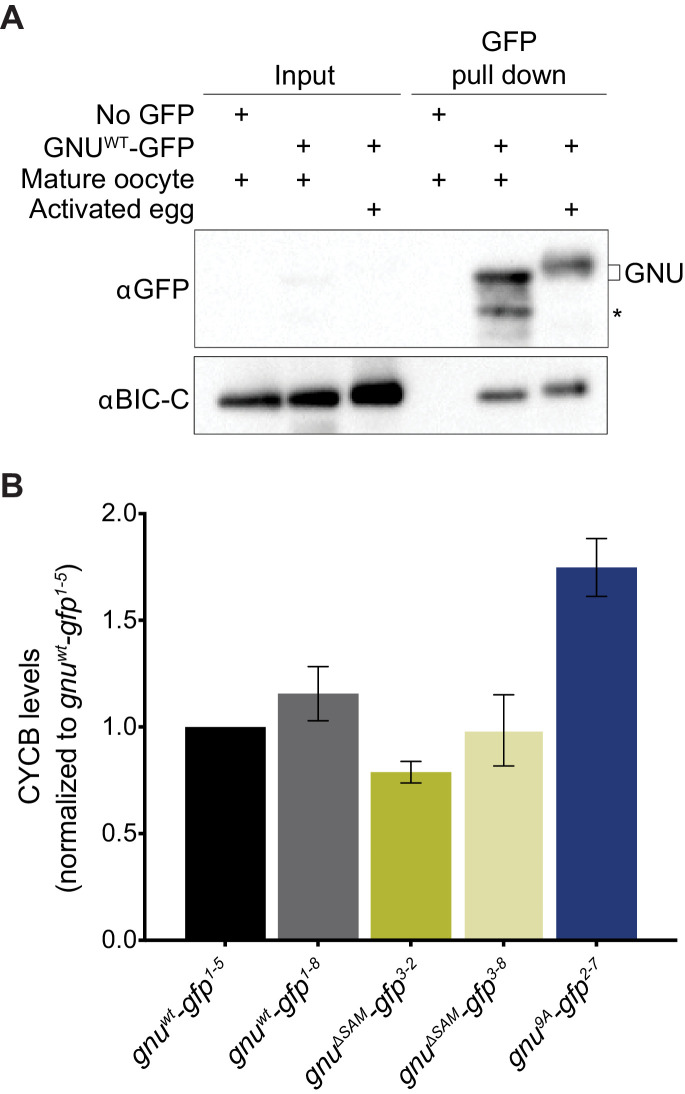
Figure 6. Experimental test for model that RNP granule localization of GNU prevents activation of PNG.
(A) BIC-C and GNU remain physically associated after egg activation. Anti-GFP magnetic beads were used to perform pull-downs of GNU-GFP from extracts prepared from isolated mature oocytes or in vitro activated eggs expressing gnuwt-gfp transgenes. For the analysis of activated eggs, mature oocytes were isolated and activated in vitro by incubation in hypotonic buffer for 20 min. GFP immunoprecipitations from no transgene (no GFP) mature oocyte extracts controlled for interactions with the beads or GFP tag. GNU-GFP pull-down from both mature oocyte or activated egg extracts results in immunoprecipitation of BIC-C. The asterisk marks a GNU-GFP degradation product we often observe in immunoprecipitations from mature oocytes. (B) Deletion of the SAM domain in GNU does not increase levels of CYCB in mature oocytes. Mature oocytes were isolated from gnu305 homozygous females expressing gnuwt-gfp, gnuΔSAM-gfp, or gnu9Α-gfp transgenes. The levels of CYCB and αTUB were examined by immunoblot. Two independent lines were analyzed for each transgene, except for gnu9Α-gfp for which only one line was analyzed. Levels of CYCB were quantified and normalized to TUB levels. The graph shows normalized levels of CYCB relative to gnuwt-gfp1-5 oocytes. Error bars correspond to SEM, and each bar represents five biological replicates. CYCB levels were not significantly different between oocytes from the two gnuwt-gfp lines (paired t-test, p=0.2855). No significant difference was observed between gnuwt-gfp and gnuΔSAM-gfp3-8 (paired t-test, p=0.9281), but the CYCB levels in gnuΔSAM-gfp3-2 oocyte were significantly lower than in gnuwt-gfp1-5 oocytes (paired t-test, *p=0.0137). Levels of CYCB in gnu9Α-gfp2-7 oocytes are significantly higher compared to gnuwt-gfp (paired t-test, **p=0.0053).