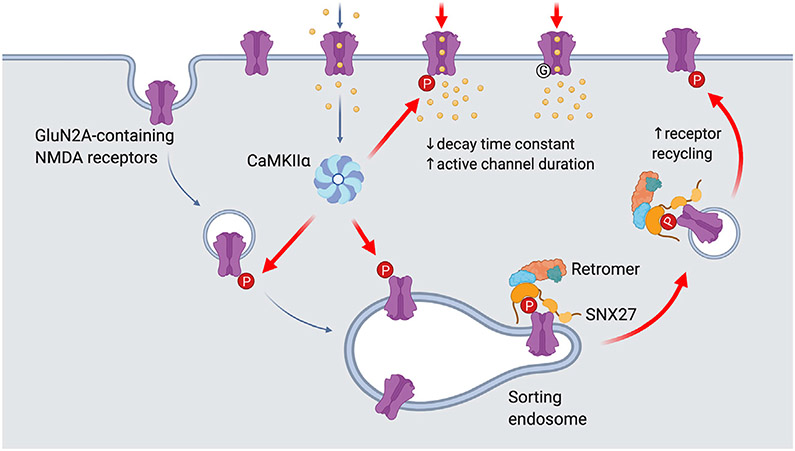
Figure 7. Proposed model for the role of Ser-1459 phosphorylation in regulating NMDAR functions.
During LTP, NMDAR-dependent influx of Ca2+ into the postsynaptic compartment activates the protein kinase CaMKIIα. Phosphorylation of GluN2A at Ser-1459 by CaMKIIα in the endocytic vesicles and/or sorting endosomes promotes its interaction with the SNX27-retromer complex, thereby enhancing the rate of GluN2A recycling back to the plasma membrane. CaMKIIα can also phosphorylate existing GluN2A-containing NMDARs on the surface and affect the gating of these ion channels by prolonging the duration of single channel opening, which in turn leads to enhanced NMDAR current density and Ca2+ influx. Importantly, the GluN2A S1459G variant, which is associated with epilepsy, also displays augmentation in synaptic NMDAR currents due to a decrease in the receptor deactivation kinetics and an increase in active channel duration. Image was created with BioRender.com.