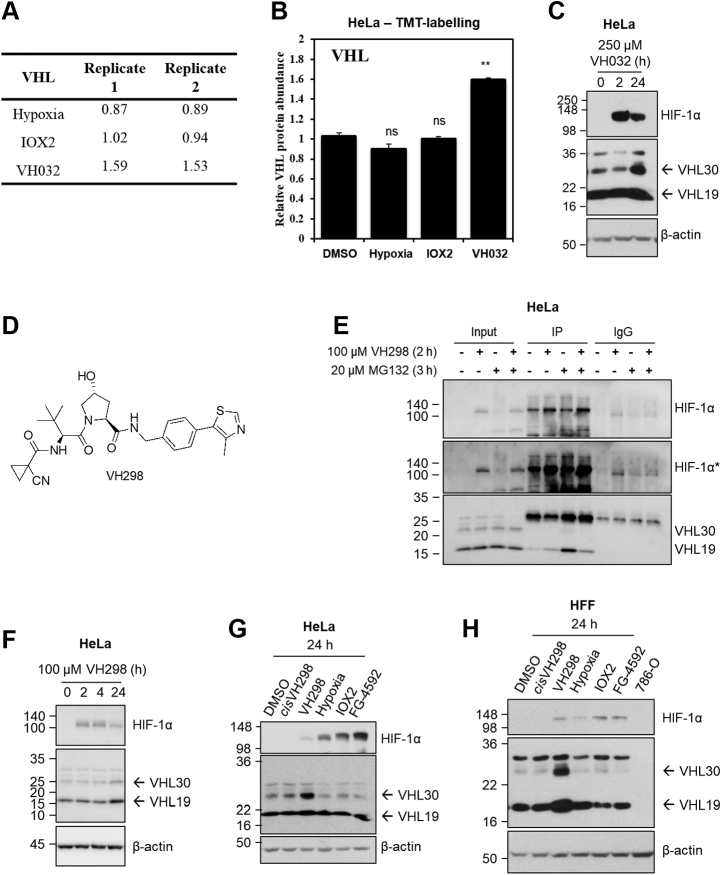
Figure 2.
VHL protein levels increase in the presence of VHL inhibitors.A, relative abundance and B, graph depicting relative VHL protein abundance with a false discovery rate <0.01 comparing with DMSO control for hypoxia, IOX2, and VH032 treatments after 24 h. B, data are presented as means + SD from proteomic analysis (TMT labeling) of two independent biological experiments. Two-tailed Student's t test was performed to calculate p values, and levels of significance are denoted as follows: ∗∗0.001 < p < 0.01 and, ns: p > 0.05. HeLa cells were treated with (C) 250 μM VH032 or (F) 100 μM VH298 for indicated time. D, chemical structure of VH298. E, coimmunoprecipitation on lysates from HeLa cells treated with vehicle DMSO (0.5% for 3 h), VH298 (100 μM for 2 h), MG132 (20 μM for 3 h), or VH298 and MG132 (100 μM VH298 for 2 h and 20 μM MG132 for 3 h) before lysis. About 300 μg of protein were used to immunoprecipitate with the 2 μg HIF-1α antibody (Santa Cruz; sc-53546). Mouse immunoglobulin G (IgG; 2 μg) was used as a control. Inputs represent 10% of the starting material used per immunoprecipitation (IP). G, HeLa or (H) HFF cells were treated with 0.5% DMSO, hypoxia (1% O2), and 100 μM of indicated compounds for 24 h. 786-O cell lysate was loaded in (H) as negative control for VHL bands. Protein levels were analyzed by immunoblotting using antibodies against HIF-1α, VHL, and β-actin, which acted as a loading control. HIF-1α∗ denotes longer exposure. The blots shown are representative of three independent experiments. DMSO, dimethyl sulfoxide; HFF, human foreskin fibroblast; HIF-1α, hypoxia-inducible factor-1α; VHL, Von Hippel–Lindau.