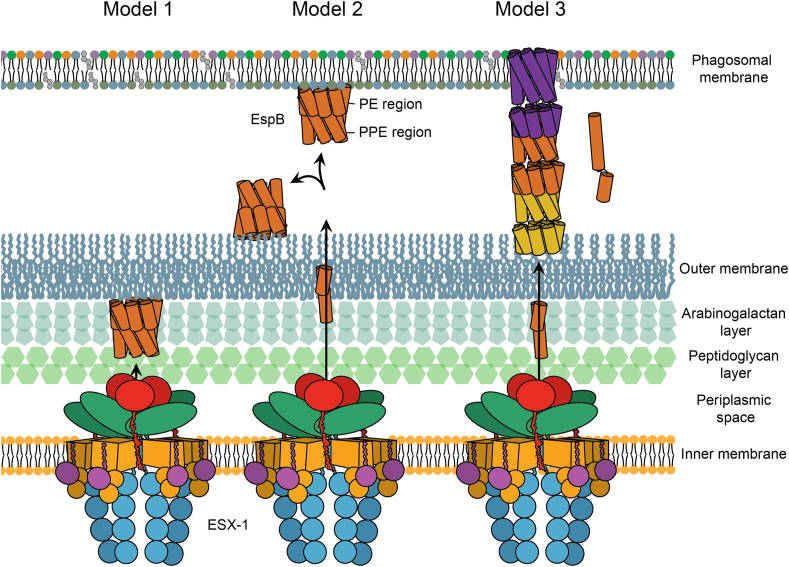
Fig. 7.
Putative pathways for the oligomerisation of EspB. In Model 1, EspB is cleaved in its C-terminus by the protease MycP1 in the periplasm of mycobacteria leaving hydrophobic residues to insert into the outer membrane; an increase in the local concentration on the membrane leads to oligomerisation of EspB. In model 2, secretion of EspB across the double membrane after MyP1 cleavage allows the protein to bind to either the phagosomal membrane or the external part of the outer membrane. In model 3, after cleavage in the periplasm and secretion to the exterior of the bacterium, EspB goes through a conformational change dissociating the PE and PPE domains and exposing hydrophobic residues that would allow the insertion into the membrane; while the PPE gets embedded into the membrane in an oligomeric form, the respective PE is able to interact with the PPE of a second molecule forming a tubular structure. Different colours are used for each heptamer-subunit. Regardless of what oligomerisation pathway EspB follows, oligomerised EspB is hypothesised to form part of the larger machinery that completes the inner-membrane complex of ESX-1.