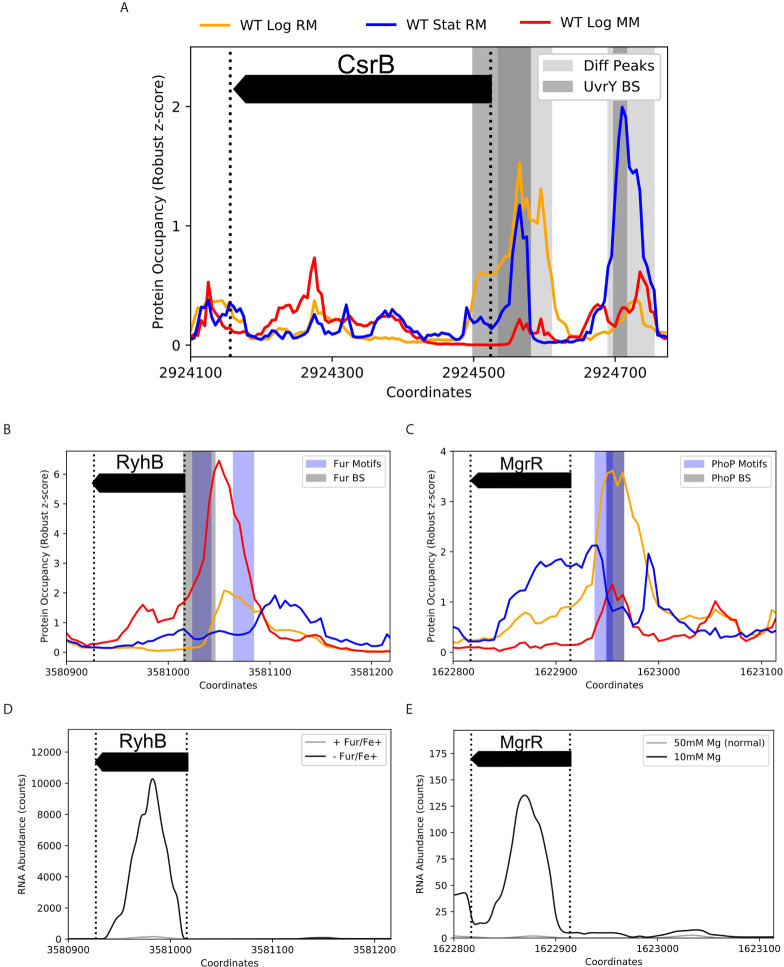
Figure 4.
Coupling IPOD-HR with FIMO captures known condition-specific protein occupancy upstream of sRNA promoter regions. Protein occupancies (PO) corresponding to 3 environmental conditions are displayed by line color (top) for K-12 samples collected in log or stationary phase grown in rich or minimal media (RM, MM). Shaded regions correspond to differential PO peaks (light grey), known binding sites (BS, grey), or FIMO-identified motifs (Motifs, blue), as listed. (A) Two differential PO peaks within -250 to +10 (with respect to TSS) of sRNA CsrB were identified. These regions overlap two previously-identified binding sites of UvrY (DNase I Footprinting: -192 to -174, ChIP-exo: -222 to -142 (not shown) and -56 to +25) (Zere et al., 2015). The binding of UvrY is known to activate CsrB transcription, however, coordination between sites is not well understood. Interestingly, the two peaks differ in presence between log and stationary phase, and between RM and MM, suggesting varying UvrY modes of binding. A UvrY consensus sequence was not in any of the tested motif databases, and therefore could not be captured by the FIMO search. B/C. PO of approximately [-200 to +10] nucleotides of the sRNAs RyhB (B) and MgrR (C) with documented BS and FIMO captured motifs of the iron-responsive Fur regulator [EMSA: -30 to +1 (Chen et al., 2007)] and the cation-responsive PhoP regulator [consensus motif identification: -52 to -36 (Moon and Gottesman, 2009)], respectively. (D) RNA-seq comparison between a fur deletion and wildtype in the presence of iron (Seo et al., 2014) supports the role of Fur repressing RyhB transcription, enabling Fur-RyhB to be captured as a high-confidence DBP-sRNA pair by the data-mining approach. (E) MgrR is highly expressed under magnesium deprivation (McClune et al., 2019). As PhoP is known to activate transcription in response to magnesium deprivation, among other divalent cation limitations, it is likely that IPOD-HR data captured calcium-dependent differential PhoP occupancy between RM (4µM CaCl2) and MM (400µM CaCl2) (C).