Abstract
Background:
Adolescent major depressive disorder (MDD) is a significant health problem, associated with substantial morbidity, cost, and mortality. Depression is a significant risk factor for suicide, which is now the second leading cause of death in young people. Up to twenty per cent of adolescents will experience MDD before adulthood, and while a substantial proportion will improve with standard-of-care treatments (psychotherapy and medication), roughly one third will not.
Methods:
Here, we have reviewed the literature in order to discuss the concept of treatment-resistant depression (TRD) in adolescence, examine risk factors, diagnostic difficulties, and challenges in evaluating symptom improvement, and providing guidance on how to define adequate medication and psychotherapy treatment trials.
Results:
We propose a staging model for adolescent TRD and review the treatment literature. The evidence base for first- and second-line treatments primarily derives from four large pediatric clinical trials (TADS, TORDIA, ADAPT, and IMPACT). After two medications and a trial of evidence-based psychotherapy have failed to alleviate depressive symptoms, the evidence becomes quite thin for subsequent treatments. Here, we review the evidence for the effectiveness of medication switches, medication augmentation, psychotherapy augmentation, and interventional treatments (i.e., transcranial magnetic stimulation, electroconvulsive therapy, and ketamine) for adolescent TRD. Comparisons are drawn to the adult TRD literature, and areas for future pediatric depression research are highlighted.
Conclusions:
As evidence is limited for treatments in this population, a careful consideration of the known risks and side effects of escalated treatments (e.g., mood stabilizers and atypical antipsychotics) is warranted and weighed against potential, but often untested, benefits.
Keywords: Depression, major depressive disorder, psychopharmacology, psychotherapy
Introduction
Major depressive disorder (MDD) is a potentially lethal problem and a major public health concern in young people. The yearly prevalence of adolescent depression is estimated to be between 4% and 8% (Lewinsohn, Hops, Roberts, Seeley, & Andrews, 1993), with the lifetime prevalence approaching twenty per cent by the end of the teenage years in the United States (Kessler, Avenevoli, & Ries Merikangas, 2001; Lewinsohn, Clarke, Seeley, & Rohde, 1994; Lewinsohn et al., 1993; Williams, O’Connor, Eder, & Whitlock, 2009). Pediatric depression is associated with increased risk of poor social and academic functioning, early pregnancy, physical illness, and substance abuse (Fergusson & Woodward, 2002; Keenan-Miller, Hammen, & Brennan, 2007; Weissman et al., 1999), and depression that begins in adolescence is associated with persistence into adulthood, with more severe clinical courses compared to later-onset MDD (Dunn & Goodyer, 2006). Depression increases the risk of suicide, now the second leading cause of death in 10- to 34-year-olds (CDC, 2017), by thirty-fold (Brent et al., 1988), and the overall financial burden of depression in society is considerable, estimated to be $210 billion in the United States in 2010 (Greenberg, Fournier, Sisitsky, Pike, & Kessler, 2015), and pediatric neuropsychiatric disorders are considered the most substantial contributor to the worldwide burden of disease in youth due to productive time lost to disability (Gore et al., 2011; World Health Organization, 2019). Thus, identifying MDD and treating it effectively in pediatric patients are critical.
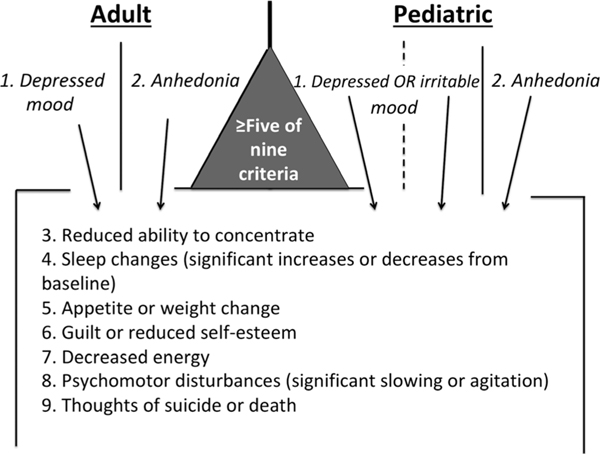
The diagnosis of MDD in adolescents relies on fulfilling five of nine Diagnostic and Statistics Manual (DSM)-5 criteria during a 2-week period (one of which must be one of the two cardinal criteria), and this constellation of symptoms must be associated with significant functional impairment (APA, 2013). These criteria are essentially the same as those used for adults (Figure 1), save for the allowance of irritability rather than strictly depressed or anhedonic mood as one of the first criteria.
Figure 1.
Differences in adult and pediatric depression diagnosis. A diagnosis requires fulfilling 5 of the 9 criteria listed, and at least one must be one of the two cardinal symptoms (listed first in the figure). These cardinal criteria serve as ports of entry to the diagnosis. A key difference between adult and pediatric diagnoses is the mood symptom, where sad or depressed mood is the criterion in adults, but sad, depressed, or irritable mood meets criteria in children. The allowance of irritability effectively creates a third port of entry to this diagnosis
These criteria also allow for considerable heterogeneity in presentation while still falling under the diagnosis of MDD, and subtypes of MDD within the broader construct surely exist (Beijers, Wardenaar, van Loo, & Schoevers, 2019). Despite having nearly identical diagnostic criteria to adults, there may be some phenomenological differences between adolescent and adult depression, with adolescents more frequently experiencing neurovegetative and somatic symptoms compared with adults, and less commonly reporting anhedonia or concentration difficulties (Nardi, Francesconi, Catena-Dell’osso, & Bellantuono, 2013; Rice et al., 2019). There may also be significant biological differences between pediatric and adult-onset MDD (Kaufman, Martin, King, & Charney, 2001). There is also evidence for substantial heterogeneity in depression which has raised concerns about whether it represents one etiologically distinct entity (Stringaris, 2017).
Guidelines exist regarding first-line treatments once a pediatric MDD diagnosis is made, largely informed by four large pediatric depression clinical trials: the Treatment of Adolescents with Depression Study [TADS (N = 439; March et al., 2004)], Treatment of Resistant Depression in Adolescents [TORDIA (N = 334; Brent et al., 2008)], Adolescent Depression Antidepressants and Psychotherapy Trial [ADAPT (N = 208; Goodyer et al., 2008)], and Improving Mood with Psychoanalytic and Cognitive Therapies [IMPACT (N = 465; Goodyer et al., 2017)] trials. A recent Practitioner Review in this journal summarizes well the current state of initial screening, assessment, and treatment (especially psychotherapy) of adolescent depression (Goodyer & Wilkinson, 2019). Both the National Institute of Clinical Excellence (NICE) guidelines (National Institute for Health & Care Excellence, 2019) and the American Academy of Child and Adolescent Psychiatry (AACAP) Depression practice parameters (Birmaher et al., 2007) emphasize confidentiality, psychoeducation, family engagement, school involvement, a thorough risk assessment for harm to self or others, and brief psychotherapy or supportive care for pediatric patients with only mild depressive symptoms. For adolescents with moderate-to-severe depression, both guidelines also recommend an evidence-based psychotherapy [e.g., cognitive-behavioral therapy (CBT)], and/or fluoxetine, although the choice of sequential versus combined treatment is left to the practitioner to decide. The NICE guidelines make an explicit recommendation to prescribe fluoxetine only in conjunction with psychotherapy (National Institute for Health & Care Excellence, 2019), whereas the AACAP practice parameter is more lenient with fluoxetine alone as a reasonable strategy. The NICE guideline recommendation to only prescribe medication in conjunction with current psychotherapy may be difficult to implement in health systems where pediatric depression is common and therapists scarce. Whether or not children are in concurrent therapy, patients started on antidepressant medication should be closely monitored given the morbidity and mortality associated with pediatric MDD.
The evidence base narrows considerably for adolescents who do not respond to these first-line treatments, limited in terms of randomized controlled trials exclusively to the TORDIA trial, which examined the impact of a medication switch and the added benefit of CBT (Brent et al., 2008). For adolescents who have not responded to this second medication and psychotherapy, a rigorous evidence base of any kind is lacking, making it challenging to formulate recommendations or treatment plans, and necessary to understand the adult TRD evidence base regarding augmentation strategies.
Thus, the current goal is to examine the concept of treatment resistance in adolescent depression in order to propose a working definition, as well as provide guidance on how to evaluate and therapeutically approach to these patients. Here, we review the process of diagnostic assessment, prior treatment evaluation, and the strengths and limitations of the pediatric MDD evidence base. We summarize and draw on the adult TRD literature and compare pediatric studies as we are able. Understanding the risk factors and presentation features of adolescent TRD cases will allow better risk stratification and resource allocation, with a goal of having high-risk cases more quickly find subspecialty care and more aggressive treatment.
Risk factors and diagnostic challenges
A key step in identifying cases with the potential for treatment resistance is to recognize the risk factors that portend poor responses to our current psychotherapies and SSRIs. Risk factors can be stratified into medical, psychiatric, and social categories. Medical risk factors include certain genetic variants [e.g., FKBP5 (Brent et al., 2010) and the short serotonin transporter polymorphism (Kronenberg et al., 2007)] and rapid metabolism profiles (Axelson et al., 2002). It is also important to take a thorough medical history when evaluating patients with depression in order to rule out or correct nonpsychiatric causes of mood disturbance [e.g., thyroid abnormalities (Joffe, 1997)]. Psychiatric risk factors for poor therapeutic responses include anhedonia (McMakin et al., 2012) and subsyndromal manic symptoms (Maalouf et al., 2012); the use of the sleep aid trazodone has also been associated with poorer SSRI responsiveness in clinical trials, especially when co-prescribed with fluoxetine (Shamseddeen et al., 2012). It remains unclear whether this association is fully attributable to confounding by indication – insomnia itself is a predictor of poor antidepressant response (Emslie et al., 2012), or whether trazodone itself is associated with poor antidepressant response. Social or familial factors that are associated with poor treatment responses include early adversity or current trauma (Nanni, Uher, & Danese, 2012; Shamseddeen et al., 2011), family conflict (Rengasamy et al., 2013), parental depression (Swartz et al., 2016; Weissman et al., 2006), being bullied (van Geel, Vedder, & Tanilon, 2014), and being a member of a sexual or gender minority group (Russell & Fish, 2016). The presence of one or more of these factors can alert mental health workers to a higher likelihood of case complexity, as well as provide targets for intervention. For example, a tactful referral of depressed parents to their own treatment may be used as a means of helping their child. In families with a high degree of conflict, family therapy should remain part of the longer-term treatment plan, but it may be difficult for adolescents in the midst of a severe depressive episode to meaningfully participate (Trowell et al., 2007).
Diagnosis can also be challenging when there is significant syndromic overlap of specific depressive symptoms with other DSM-5 diagnoses. For example, concentration difficulties can be seen in both MDD and a defining feature of attention-deficit/hyperactivity disorder (ADHD; APA, 2013). Thus, the presence of the symptom is not the end of the story, but rather an invitation to evaluate the time course of individual symptoms and their co-occurrence with other symptoms in arriving at the most accurate and parsimonious diagnosis. More long-standing concentration problems that were present during periods of euthymia would thus be more consistent with ADHD, although attention problems could be further exacerbated during a superimposed depressive episode. An accurate formulation of concentration symptoms is important, as treating impairing ADHD symptoms may improve social and scholastic function, leading to improved self-esteem and mood.
Irritability is a symptom that can also cause diagnostic confusion and disagreement among providers (Stringaris, Vidal-Ribas, Brotman, & Leibenluft, 2018). Although longitudinal studies have not borne out a relationship between irritability and subsequent bipolar disorder (Stringaris, Cohen, Pine, & Leibenluft, 2009), particularly intense or persistent irritability has a legacy of raising suspicion for underlying bipolar disorder (Serra et al., 2017) or disruptive mood disorder with dysregulation (DMDD), even if other criteria for those disorders [e.g., decreased need for sleep in the case of bipolar disorder, or recurrent, severe temper outbursts for DMDD (APA, 2013)] are absent. The neurobiology, differential diagnosis, and treatment of irritability in children have been well-described elsewhere (Stringaris et al., 2018). In longitudinal studies of persistently irritable children, this irritability phenotype is more likely to portend conversion to MDD (Rice et al., 2017; Vidal-Ribas, Brotman, Valdivieso, Leibenluft, & Stringaris, 2016), rather than bipolar disorder (Stringaris et al., 2009, 2010). The worry about bipolar disorder, coupled with community concerns regarding behavioral activation syndromes as potential SSRI side effects (Strawn, Welge, Wehry, Keeshin, & Rynn, 2015), can often prevent these children from receiving a reasonable trial of first-line depression pharmacotherapy and can result in the unwarranted prescription of medications with higher side effect burdens (mood stabilizers and antipsychotics; Correll & Blader, 2015). In irritable patients with other clear features of depression and the absence of a history of genuine mania (i.e., fulfilling all of the DSM-5 criteria for a manic episode), we recommend treating patients as MDD until proven otherwise, which entails a trial of an SSRI, typically fluoxetine, for 8 weeks at a therapeutic dose. In fact, recent evidence demonstrates that severe irritability may be reduced using SSRIs (Towbin et al., 2019). While estimates vary (Offidani, Fava, Tomba, & Baldessarini, 2013), the risk of conversion to mania that meets full DSM-5 criteria with SSRIs is a rare event that appears often coincidental rather than caused by changes in medications (Baldessarini et al., 2013). We recommend tolerating some degree of side effects, including activation, in order to complete a full antidepressant trial as the alternative medication options for MDD have a weaker evidence base and increased side effect burden. Additionally, there are very little data in the literature that examines how activation, mania, and increases in suicidal ideation change with continued SSRI treatment.
It is important to consider other common comorbidities in adolescent patients with MDD and to screen for these in order to obtain the most complete diagnostic formulation. For example, trauma serves as a potent risk factor for adolescent depression and poor responses to treatment (Shamseddeen et al., 2011), and also creates a risk for post-traumatic stress disorder (PTSD). Assessment of the presence and degree of PTSD symptoms is important, as severe PTSD symptoms may enhance domains of depressive symptomatology (Nichter, Norman, Haller, & Pietrzak, 2019). For example, sleep disturbances associated with MDD may be exacerbated by trauma-related nightmares, and the ability to engage in and enjoy social activities may be inherently compromised by hypervigilance.
Anxiety disorders are also commonly comorbid in pediatric MDD (Birmaher et al., 2007; Lewinsohn et al., 1993). Although anxiety disorders respond to similar first-line medications as pediatric depression [e.g., SSRIs (Dobson, Bloch, & Strawn, 2019)], evidence-based psychotherapy (particularly cognitive-behavioral therapy) differs substantially between the two conditions. The consequences of pediatric anxiety disorders (e.g., social isolation in a teenager with social anxiety) can both be a precipitant of a depressive episode and also be worsened by depression leading to a vicious negative feedback cycle.
Adolescent MDD is also associated with an increased risk of a substance use disorders (SUD; Weissman et al., 1999). SUDs are associated with more severe depressive symptom profiles in adults (Carton et al., 2018) and decreased likelihood of remission in depressed adolescents (Emslie et al., 2010). A frank, nonjudgmental inquiry into illicit or recreational drug use, coupled with a motivational interviewing approach, can be useful in determining the extent of any substance use and in harm reduction (D’Amico et al., 2018). Psychoeducation regarding the insidious relationship between mood disturbances and substance abuse should be provided, and the degree of drug or alcohol use should be taken into careful consideration during treatment planning.
Depression also increases the risk for the presence of eating disorders (e.g., anorexia, bulimia), and vice versa, particularly in younger populations (Puccio, Fuller-Tyszkiewicz, Ong, & Krug, 2016). Thus, it is important to screen for these disorders, particularly in patients with high depressive symptomatology in the appetite and self-esteem domains. Nutritional deficiencies associated with eating disorders commonly manifest similar to depression symptoms (Bodnar & Wisner, 2005).
One additional common area of diagnostic uncertainty, both in adult and in pediatric chronic depression populations, is that of co-occurring personality disorders, specifically the concern of borderline personality disorder (BPD) when nonsuicidal self-injury is present (Turner et al., 2015). While the diagnosis of personality disorders prior to full-fledged adulthood has been controversial (Miller, Muehlenkamp, & Jacobson, 2008), DSM-5 allows for this diagnosis in adolescence, requiring five of nine diagnostic criteria to be met (APA, 2013). Complicating the diagnosis of BPD in adolescence is that several criteria could be interpreted to varying degrees as normative features of adolescence or in keeping with adolescent developmental tasks. For example, increased impulsivity and risk taking are features of this developmental period that are shared across mammalian species (Spear, 2000). Unstable self-image can also be a normative finding as teenagers are actively building a sense of self and consolidating an adult identity (Gilmore & Meersand, 2013; Sharp & Wall, 2018). Finally, intense interpersonal relationships and defensive styles of idealization and devaluation are also somewhat common in normative adolescence (Gilmore & Meersand, 2013). Areas of potential overlap with pediatric MDD diagnoses include irritability and affective instability (already a challenging symptom domain diagnostically) and recurrent suicidality or self-harm. Some diagnostic distinction may be reached with attention to time course (symptoms persisting for greater than 1 year in BPD), recurrent dysfunction across several affective and social contexts, and attention to cognitive symptoms of BPD, such as dissociation or paranoid ideation under stress (Guile, Boissel, Alaux-Cantin, & de La Riviere, 2018). There are multiple validated instruments for adolescent BPD (Guile et al., 2018; Sharp, Ha, Michonski, Venta, & Carbone, 2012); however, in practice, adolescents who meet criteria for BPD in both outpatient and inpatient settings almost invariably have comorbid mood disorder diagnoses (Chanen, Jovev, & Jackson, 2007; Kaess et al., 2013).
When comorbid or overlapping disorders are present, the question arises as to which disorder to prioritize in treatment, or whether to tackle multiple problems simultaneously. When looking strictly at psychotherapy trials, targeting multiple problems simultaneously leads to a dramatic reduction in the effect size of therapy, rendering it statistically equivalent to zero (Weisz et al., 2017). Thus, when it comes to psychotherapy it is likely best to start with a relatively narrow focus and approach one problem at a time (Craske et al., 2007). Generally, it is helpful to prioritize early in treatment the symptoms that (a) patient is most amenable to targeting, (b) patient reports experiencing the greatest impairment from, and (c) that the therapist perceives (and the evidence suggests) is most amenable to therapy. For instance, the benefits in terms of effect size of evidence-based psychotherapy (Weisz et al., 2017) are higher for pediatric anxiety disorders than depression; thus, it may often be advisable to start psychotherapy targeting particularly impairing anxiety symptoms with target CBT exercises. In terms of pharmacotherapy, SSRIs remain the first line for depression, anxiety, and obsessive–compulsive disorders, with evidence for post-traumatic stress disorder in adults but not pediatric patients (Cohen, Mannarino, Perel, & Staron, 2007; Dwyer & Bloch, 2019). SSRIs are also recommended for mild-to-moderate mood symptoms in adult patients with BPD (Gunderson & Links, 2014). Thus, the same medication may reasonably address multiple comorbidities. SSRIs may be particularly beneficial in depressed adolescents with comorbid anxiety (Davey et al., 2019).
Evaluating treatment
Measuring efficacy
The first component required to evaluate treatment response is to agree on what constitutes a response and to whom; issues of measurement and informant are far from trivial. There has long been debate regarding the validity of parent versus child reports for internalizing disorders (Moretti, Fine, Haley, & Marriage, 1985). While some components of depressive syndromes are amenable to objective measurement (e.g., hours of sleep, amount of food consumed, and grades in school), other features (e.g., feeling sad and loss of pleasure) are inherently more subjective to the child’s experience. While parents (and clinicians) can infer mental states based on behavior or affect, it is not immediately clear whose report to prioritize if a child reports feeling depressed but does not appear depressed. On the one hand, one might imagine a child who feels quite depressed, but is able to effectively mask those feelings publicly, in which case, we might prioritize the adolescent’s report when considering depression severity. On the other hand, it is not uncommon for improvement in neurovegetative symptoms and affect changes to occur in the course of depressive recovery prior to improvement in subjective senses of well-being (Keilp et al., 2018), and thus, here one might prioritize parent or teacher reports. A recent meta-analysis of psychotherapy trials highlighted the risks of relying on solely one informant, particularly in MDD (Weisz et al., 2017). While informant (parent, child, or teacher) did not significantly influence effect size for anxiety trials, in depression trials, child report yielded the largest effect (effect size of 0.32), while parent report yielded a nonsignificant effect (effect size of 0.15), and teacher reports deemed therapy-treated adolescents significantly worse than control-treated (effect size of 0.41; Weisz et al., 2017). Nonagreement across informants is unfortunately rather common in child mental health (De Los Reyes et al., 2015), adding an additional challenge to diagnosis and efficacy assessment.
Thus, it appears important that multiple informants play a role in evaluations, at the very least the adolescent and one parent, which is the standard in Child Psychiatry subspecialty practice (De Los Reyes et al., 2015). The time required, however, can make multiple interviews challenging in primary care settings, where MDD diagnoses are often first made (Williams et al., 2009). Standardized clinical instruments such as the clinician-administered 17-item Children’s Depression Rating Scale-Revised (CDRS-R; Mayes, Bernstein, Haley, Kennard, & Emslie, 2010; Poznanski et al., 1984) and the self-report Mood and Feelings Questionnaire (MFQ; Daviss et al., 2006; long version of 33 questions, short version of 13 questions) include both parent and child versions, and rely on the clinician to synthesize this information to determine the most accurate clinical picture. The CDRS-R is used as the gold standard instrument in pediatric MDD trials, with a reduction in greater than fifty per cent from baseline considered a significant response (Poznanski et al., 1984). Recent data in adult psychiatry suggest that implementing standardized instruments and measurement-based care into the clinic is both feasible, and leads to better outcomes in MDD compared with standard care (Guo et al., 2015). Some depression rating scales such as the Montgomery–Asberg Depression Rating Scale (MADRS) also have strong data of validity, especially in older adolescent populations (Jain et al., 2007; Torres Soler et al., 2018).
Functional outcomes are also important to consider, including quality of social interactions, academic performance, perceived enjoyment of activities, age-appropriate participation in activities of daily living, health system utilization, and suicidality or suicidal behavior. Adolescent MDD is associated with poorer adult functional outcomes, including failure to complete high school [odds ratio (OR) 1.76, 95% confidence interval (CI) 1.29–2.39], unplanned pregnancy (OR 1.38, 95% CI 1.06–1.81), and unemployment (OR 1.66, 95% CI 1.29–2.14; Clayborne, Varin, & Colman, 2019). In adult studies, functional impairment often persists even when patients remit by clinical symptom criteria (Coryell et al., 1993), particularly in more treatment-resistant populations (Trivedi et al., 2013). One imagines that for patients, a true recovery implies recovering an ability to lead a reasonably satisfying and productive life, underscoring the importance of functional outcomes. The efficacy of treatments in clinical trials, however, does not always align between clinical and functional outcomes, as patients who show clinical symptom reduction do not always show improvements in functional outcomes, and vice versa (Lam et al., 2013).
Thus, issues of measurement are complicated, perhaps particularly so in Child Psychiatry, where careful attention is paid to multiple informants and vested parties involved (child, parents, teachers), and a high degree of comorbidity (Birmaher et al., 2007; Lewinsohn et al., 1993) and diagnostic uncertainty exists. In addition to a careful, comprehensive, multi-informant-based formulation, we recommend choosing at least one clinical instrument to be given at regular intervals in order to gauge progress. Self-report measures, such as the Mood and Feelings Questionnaire (Daviss et al., 2006) and the Children’s Depression Inventory (Kovacs, 1985), are perhaps the least cumbersome and could be completed prior to an appointment at home or in the waiting room. While clinician scored measures and additional scales relating to functional outcomes would contribute important information, choosing arguably the simplest scales to implement may facilitate an easier transition to measurement-based care, which has been shown to improve outcomes in the adult MDD populations (Guo et al., 2015).
Defining adequate treatment
Pharmacotherapy.
Although only two antidepressants (fluoxetine and escitalopram) are FDA-approved for pediatric MDD, and current NICE guidelines recommend utilizing fluoxetine as the first-line pharmacological treatment in this population (National Institute for Health & Care Excellence, 2019), there is no strong evidence base to suggest that any particular SSRI agent is more effective than any other for pediatric depression (Dwyer & Bloch, 2019). Meta-analyses comparing the efficacy of different SSRI agents in pediatric depression have typically failed to demonstrate a difference between individual medications within the SSRI class (Varigonda et al., 2015). A network meta-analysis that excluded treatment-resistant cases identified fluoxetine as having the most robust efficacy versus placebo in pediatric depression studies, but notes that quality of evidence comparing other compounds was very low (Cipriani et al., 2016). Although there have been large differences in the measured efficacy of SSRIs compared to placebo between trials, the major NIMH-sponsored adolescent depression trial, TADS, showed that SSRIs (fluoxetine in this case) were quite effective with a number needed to treat (NNT) of 4 over the acute (12-week) phase (Kennard, Silva, et al., 2009). The NNT is the inverse of the absolute risk reduction and represents the number of patients that would need to receive the medication in order to obtain one antidepressant response compared to receiving placebo, with lower NNTs implying more effective interventions (Cook & Sackett, 1995). Many industry-sponsored trials for pediatric MDD had large placebo response rates (~60%), which resulted in smaller between-group differences, and estimates of NNT of closer to 12 (Walkup, 2017), which has introduced substantial variability in meta-analyses that include all trials (Cipriani et al., 2016). It has been suggested that methodological differences between trials may be responsible for the differences in the measured treatment effects of antidepressant medications (discussed in Section Strategies for treatment-resistant depression; Walkup, 2017). Despite the large range in the estimated efficacy of pediatric antidepressant agents, systematic reviews and meta-analyses regarding SSRI pharmacotherapy have largely demonstrated that they are effective for pediatric MDD (Bridge et al., 2007; Hammad, Laughren, & Racoosin, 2006; Reyes, Panza, Martin, & Bloch, 2011; Wallace, Neily, Weeks, & Friedman, 2006; Whittington et al., 2004), with greater separation from placebo (i.e., bigger effect sizes) in patients who are more severely ill at the beginning of the trial (Bridge, Birmaher, Iyengar, Barbe, & Brent, 2009). SSRIs as a class provide around a 25% greater chance of responding over the short-term when compared to placebo and have a NNT of 10 (Bridge et al., 2007; Wallace et al., 2006) although it is clear that earlier treatment estimates of SSRI efficacy were exaggerated by publication bias and time-lag bias in the publication of negative trials (Reyes et al., 2011; Whittington et al., 2004).
Current NICE treatment recommendations in pediatric depression suggest that an adequate trial of an antidepressant agent is at least 8 weeks of treatment at or above the minimally recommended dose (National Institute for Health & Care Excellence, 2019). Table 1 depicts the recommended starting doses and the typical dose range in both pediatric and adult patients of commonly utilized antidepressants in pediatric and adult populations. Many medications currently prescribed for pediatric depression have minimal evidence of efficacy. The current AACAP Practice Parameter recommends that, ‘patients should be treated with adequate and tolerable doses for at least 4 weeks. Clinical response should be assessed at 4-week intervals, and if the child has tolerated the antidepressant, the dose may be increased if a complete response has not been obtained. However, patients who are showing minimal or no response after 8 weeks of treatment are likely to need alternative treatments. Furthermore, by about 12 weeks of treatment, the goal should be remission of symptoms, and in youths who are not remitted by that time, alternative treatment options may be warranted’ (Birmaher et al., 2007).
Table 1.
First-line pharmacological treatments for adult depression and evidence of their effects in pediatric populations
| Pediatric |
Adult |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Starting dose (mg/day) | Typical dose range (mg/day) | Level of evidence in MDD | FDA indications | Starting dose (mg/day) | Typical dose range (mg/day) | Level of evidence in MDD | FDA indications | Half-life | |
| Selective serotonin reuptake inhibitors | |||||||||
| Citalopram | 10–20 | 20–40 | C | – | 20 | 40 | A | MDD | 20 hr |
| Escitalopram | 10 | 10–20 | A | MDD (12+) | 10 | 10–20 | A | MDD, GAD | 27–32 hr |
| Fluoxetine | 10–20 | 20–80 | A | MDD (8+), OCD (7+) | 20 | 20–80 | A | MDD, OCD, PD | 4–6 days |
| Fluvoxamine | 25–50 | 50–300 | C | OCD (8+) | 100–300 | 100–300 | A | OCD | 16 hr |
| Paroxetine | 10–20 | 20–60 | C | – | 10–20 | 40–60 | A | MDD, OCD, PTSD, GAD, SAD, PD | 21 hr |
| Sertraline | 25–50 | 100–200 | A | OCD (6+) | 50 | 150–250 | A | MDD, OCD, PTSD, SAD, PD | 26 hr |
| Serotonin–norepinephrine reuptake inhibitors | |||||||||
| Venlafaxine | 37.5 | 150–225 | C | – | 37.5–75 | 75–375 | A | MDD, GAD, SAD, PD | 10 hr |
| Duloxetine | 30 | 40–60 | C | GAD (7+) | 20–60 | 20–80 | A | MDD, GAD | 12.5 hr |
| Desvenlafaxine | 25 | 25–100 | C | – | 50 | 50–400 | A | MDD | 11 hr |
| Atypical antidepressants | |||||||||
| Bupropion | 100 | 150–300 | C | – | 100–150 | 150–300 | A | MDD | 21 hr |
| Mirtazapine | 7.5–15 | 15–45 | C | – | 15 | 15–45 | A | MDD | 20–40 hr |
| Vilazodone | 5 | 10–20 | C | – | 10 | 10–40 | A | MDD | 25 hr |
| Vortioxetine | 5 | 10–20 | C | – | 10 | 10–80 | A | MDD | 66 hr |
Table 1 depicts pharmacological agents approved for the treatment of adult major depressive disorder (MDD) and their current level of evidence of efficacy in children. Only two medications are FDA-approved for treatment of major depression in pediatric populations (fluoxetine and escitalopram) depicted in bold. Sertraline has additional evidence of efficacy in multiple placebo-controlled trials in pediatric populations. Levels of evidence in both adult and pediatric patients are based on grades developed from the National Guidelines Clearinghouse (AHRQ; Shekelle, Woolf, Eccles, & Grimshaw, 1999). Grade A evidence is based on meta-analysis of randomized controlled trial (RCT) data or 1 or more RCTs. Grade B evidence is based on at least 1 controlled trial that was not randomized. Grade C evidence is based on either data from nonexperimental studies or extrapolated from Grade A or Grade B evidence in a different population. Grade D is based on expert opinion or clinical experience. Medication half-life is based on available data from adult populations. For pediatric approved medications, minimal age of approval in years is depicted in parentheses. GAD, generalized anxiety disorder; MDD, major depressive disorder; mg, milligrams; OCD, obsessive–compulsive disorder; PD, panic disorder; PTSD, post-traumatic stress disorder; SAD, social anxiety disorder.
In contrast, a meta-analysis of randomized, placebo-controlled trials in pediatric depression suggests that the greatest incremental treatment gains of SSRIs compared to placebo are seen earlier in treatment and are statistically significant by 2 weeks into treatment (Varigonda et al., 2015). On average, nearly half of the treatment benefits of SSRIs over placebo are observed at 2 weeks and more than two thirds of the gains are apparent at 4 weeks of treatment (Varigonda et al., 2015). These data suggest that early treatment gains to antidepressant treatment in pediatric MDD may be strongly predictive of overall response. Previous trials in adult depression have also strongly associated early SSRI response with improved treatment response (Szegedi et al., 2009). However, given the paucity of evidenced-based pharmacological treatment options for pediatric depression, we would still recommend full 8-week or more trials of antidepressant medications whenever possible. In cases of severe depressive symptoms or persistent suicidality that have not improved at all by 6 weeks, it may be reasonable to move to another agent sooner rather than continue an additional two weeks to satisfy a full 8-week trial. The use of depression rating scales from multiple informants, rather than relying solely on unstructured child or parent report, is probably particularly important for determining how and whether specific depressive symptoms have responded to treatment (or not).
Although there are no fixed-dose trials of SSRI medications that evaluate the comparative efficacy of different doses, current treatment guidelines recommend increasing the dose of SSRI if a child has failed to achieve adequate symptom relief from initial pharmacotherapy at the minimum recommended dose after 4 weeks (Birmaher et al., 2007). This recommendation is based on meta-analysis of adult antidepressant trials that have demonstrated a significant but modest improvement in depression symptoms with higher doses of SSRI pharmacotherapy (Jakubovski, Varigonda, Freemantle, Taylor, & Bloch, 2016). Additionally, a previous trial that examined 29 children who had not responded adequately to 20 mg of fluoxetine at 9 weeks of treatment suggested that raising the dose of fluoxetine to 40–60 mg (response rate 71%) was more effective than maintaining the dose at 20 mg (response rate 36%; Heiligenstein et al., 2006).
We would therefore recommend that an adequate trial of SSRI pharmacotherapy of pediatric depression be defined as at least 6–8 weeks of treatment on the minimally recommended FDA dose. Furthermore, we would recommend that in most cases, children who do not respond adequately to this initial dose of medication should be titrated up to the maximum recommended tolerated dose to gauge treatment response. Furthermore, there is no evidence for efficacy of subtherapeutic dosing of SSRI in children in the acute phase of treatment or for relapse prevention (Jakubovski et al., 2016), and thus, we would not consider a medication trial to have officially begun from an efficacy standpoint until the therapeutic dose range is reached. Therefore, the total time a medication is prescribed may be longer than 6–8 weeks if there is a period of subtherapeutic dosing on the front end, or a period of dose uptitration after a partial response on the lagging end. Pharmacological SSRI trials where children cannot tolerate an SSRI agent due to side effects at doses below the minimally recommended dose range should be classified as ‘aborted due to tolerability’ rather than nonresponse to SSRI medications. In the event that a patient’s depression remits on a particular medication, the acute phase is followed by a continuation phase of 16 to 20 weeks with a goal of consolidating gains and preventing relapse (Birmaher et al., 2007). These phases are then followed by a maintenance phase of variable duration that depends on the child’s history and the families’ preferences and values, with generally more severe courses or protracted recoveries warranting a longer maintenance phase (Dwyer & Bloch, 2019).
Establishing adherence with medications is a very important component to the evaluation, and even in clinical trial settings, adherence can be surprisingly low. For instance, 47% of subjects in the TORDIA trial were judged clinically nonadherent as judged by missing more the 30% of the medication doses based on pill count remainders (Woldu et al., 2011). Verifying the adequacy of previous dosing and the duration of antidepressant treatment can typically be tracked through checking refill requests or intervals through the patient’s pharmacy or checking pill counts.
Psychotherapy.
Psychotherapy is another critical component of MDD treatment, and it is just as important to assess the presence, number, and quality of psychotherapy trials as it is to assess medication history. Psychotherapy as a monotherapy is recommended by both the NICE and AACAP guidelines as the treatment of choice for mild pediatric depression (Birmaher et al., 2007; National Institute for Health & Care Excellence, 2019). For MDD overall, the effect size of therapy as a standalone treatment is small (0.29) and is significantly influenced by informant, with the highest positive effects reported in the patients themselves, modest effects in parents, and negative effects in teachers (Weisz et al., 2017). CBT is the therapy with the most data in adolescent populations, studied alone or in combination with medication in three large trials: TADS (March et al., 2004), TORDIA (Brent et al., 2008), ADAPT (Goodyer et al., 2008), and compared with short-term psychoanalytic psychotherapy (STPP) and a brief psychosocial intervention in the IMPACT trial (Goodyer et al., 2017). Despite being more thoroughly studied than other specialized psychotherapies, CBT and interpersonal psychotherapy for adolescents (IPT-A) have been demonstrated equally (Weersing, Jeffreys, Do, Schwartz, & Bolano, 2017) or near equally (Eckshtain et al., 2019) clinically effective, and the IMPACT trial demonstrated equal improvement in depressed adolescents with CBT, STPP, and a brief psychosocial intervention at the end of the treatment and at a year later (Goodyer et al., 2017).
Therapy in combination with medication holds the most intuitive appeal for more severe cases of MDD, and this combination has been tested in multiple clinical trials. The initial TADS analysis found combination treatment superior to fluoxetine treatment alone (which was significantly better than both CBT alone and placebo), in most, but not all acute (6- and 12-week) outcomes (March et al., 2004). In particular, some adverse effects that were present at increased rates in the fluoxetine-alone group compared to placebo (e.g., irritability, risky behavior, and agitation) were reduced when fluoxetine was given in combination with CBT (March et al., 2004), although this mitigating influence was not replicated TORDIA, ADAPT, or IMPACT (Goodyer & Wilkinson, 2019). Secondary analyses of the TADS trial gave a more nuanced picture, with combination therapy superseding fluoxetine-alone treatment in mild and moderate cases, but showing no statistical separation from placebo in the most impaired sets of patients (Curry et al., 2006). The ADAPT trial replicated this finding in its more severe MDD cohort, showing that the addition of CBT to medication was not significantly different from medication and usual care in a moderate-to-severe depressed population (Goodyer et al., 2008). A recent study combining adolescents and young adults (15–25 years old, N = 153) with relatively severe depression examined the converse question and showed no benefit of adding fluoxetine to CBT on depression scores at 12 weeks, although anxiety scores were significantly improved by combined treatment (Davey et al., 2019). At 26 weeks, when separated by age, participants younger than 18 showed substantial reductions in MADRS in both groups [−20.5 (95% CI −25.3 to −15.7) in CBT + placebo and −21.0 (95% CI −26.2 to −15.9) in CBT + fluoxetine] and no group differences, whereas participants older than 18 showed lower overall improvements and a significant between-group difference favoring CBT + medication [193 (95% CI −22.3 to −16.3)] over CBT + placebo [−14.2 (95% CI −17.2 to −11.1)] (Davey et al., 2019). These authors suggest that in cases where CBT provides only a modest effect, medication may provide an additional benefit, obscured by a ceiling effect in the adolescents. Taken together, these trials paint a complex picture that draws into question the conventional wisdom that combined treatment is superior to either treatment alone or sequential implementation.
The larger trials also allowed the opportunity to explore different trajectories of treatment response, with subjects potentially having differential responsiveness to therapy, medication, and the combination, which could be obscured at the group level when all potential subgroups are combined. Secondary analyses of TADS (Scott, Lewis, & Marti, 2019) and TORDIA (Maalouf et al., 2012) demonstrate three distinct trajectories of change: (a) a high severity with early improvement group, (b) a high severity with no remission group, and (c) a moderate severity with slow or later-onset remission group. Group 1, approximately 10% of the TADS sample, was highly enriched for fluoxetine treatment and had little to no CBT-alone cases (Scott et al., 2019). While this group improved quickly, most still met criteria for clinical depression at six weeks, despite substantial improvement. One interpretation of this finding is that a high initial depression severity might make it difficult for subjects to fully engage in psychotherapy due to potential cognitive, attention, and motivational impairments. The symptomatic improvement at 6 weeks due to fluoxetine might allow for better engagement with subsequent psychotherapy, and thus, a sequential rather than combined approach might be more successful for this group. The subjects in the second group, who did not substantially improve, were older with higher depression severity and longer depressive episode duration (Maalouf et al., 2012; Scott et al., 2019). The third group, comprised of moderate severity patients with slow improvement, represented three quarters of the TADS sample, was enriched with CBT treatment, and many were close to remission at the 6-week assessment point (Scott et al., 2019). These data are consistent with current guidelines for psychotherapy treatment for mild or moderate pediatric MDD cases (Birmaher et al., 2007; National Institute for Health & Care Excellence, 2019).
Issues of dose are not unique to medication, and these large trials allowed for the exploration of psychotherapy dose effects, as well as teasing apart individual components of CBT that are associated with efficacy. For example, in TORDIA, achieving a minimum dose of psychotherapy (i.e., number of sessions) appears important, as subjects who attended more than nine CBT sessions had a 2.5 times greater likelihood of response compared to those with less than nine sessions (Kennard, Clarke, et al., 2009). Similar to SSRIs, early treatment gains may predict overall response, with one trial showing that the rate of improvement over the first five CBT sessions predicted the final antidepressant response (Lewis, Simons, & Kim, 2012). Secondary TORDIA analysis also showed that the most effective components of CBT were the problem-solving and social skill components, with less emphasis on behavioral activation, emotion regulation, and family-oriented work (Kennard, Clarke, et al., 2009). As with pharmacotherapy, it is also important to establish adherence, and even in clinical trials, attendance to therapy visits can be surprisingly low [e.g., in the IMPACT trial, all participants attended a median of 6–11 sessions, despite having scheduled 28 sessions for the STPP group and 20 sessions for the CBT group (Goodyer et al., 2017)]. Ways to evaluate the adequacy of adherence to a psychotherapy regimen include inquiring about attendance, asking the participant to articulate the principles of the therapy (i.e., what is the theory of the disorder and of therapeutic change), and in CBT-based therapies, inquiring about homework assignments or exposures to uncomfortable situations.
Working definitions of treatment resistance in pediatric depression
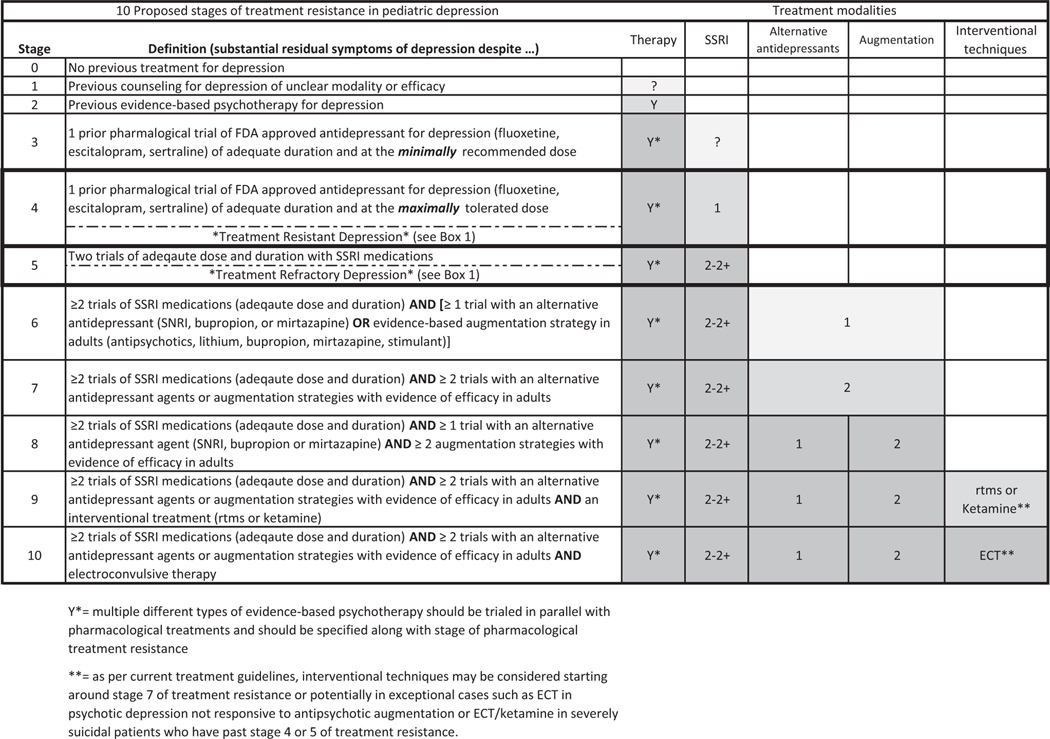
Box 1 depicts the suggested definitions for treatment response, as well as treatment-resistant depression (TRD) and treatment-refractory depression in pediatric patients, and Figure 2 depicts a 10-stage model for determining the level of treatment resistance. Multiple structured scales are available to measure and stage treatment-resistant depression in adults (Fekadu et al., 2009; Sackeim et al., 2019; Souery et al., 1999), but none have been developed to adjust to the current evidence base of treatments for depression in pediatric populations. Here, we have defined treatment resistance as having clinically significant depressive symptoms despite 1 adequate medication trial and 1 adequate psychotherapy trial, either sequentially or in combination, which corresponds to Stage 4 in Figure 2. We have defined treatment-refractory depression as having significant depressive symptoms despite 2 adequate medication trials and 1 adequate psychotherapy trial, corresponding to Stage 5 in Figure 2.
Box 1. Treatment definitions in pediatric depression. It depicts our working definition for common terms in pediatric depression. These are extrapolated based on similar guidelines for adult depression (Gaynes et al., ) but adjusted given the different levels of evidence for treatments in pediatric populations
| Term | Term Definition |
|---|---|
| Treatment-resistant depression | Clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and an antidepressant with Grade A evidence for treating depression in pediatric population (fluoxetine, escitalopram, or sertraline) |
| Treatment-refractory depression | Clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and at least 2 antidepressants with Grade A evidence for treating depression including at least 1 with Grade A evidence in pediatric populations (fluoxetine, escitalopram, or sertraline) |
| Remission | Minimal symptoms of depression after treatment |
| Response | Substantial improvement in depression symptoms with treatment. Greater than a 50% improvement in depression symptoms |
| Partial response | Modest improvement in depression symptoms with treatment. Defined as a 25%–49% improvement in depression symptoms |
| Nonresponse | Minimal-to-no improvement in depression symptoms with treatment. Defined as a less than 25% improvement in depression symptoms |
Key points
Adolescent depression is a significant public health problem associated with significant morbidity and mortality.
Nearly 40% of adolescents remain depressed after initial treatment, and over half of that population remain depressed despite switching medications or adding psychotherapy.
There is limited pediatric evidence to guide clinicians as to how to proceed therapeutically with these ‘treatment-resistant’ patients, nor is there a clear, systemized method to identify them.
We propose definitions of pediatric treatment-resistant and treatment-refractory depression, and review the evidence base regarding treatment strategies for these patients, comparing with the adult treatment-resistant literature.
We propose a staging model of treatment resistance for pediatric depression, which is relevant both for clinical practice and for recommending areas where additional research is needed.
Figure 2.
Proposed stages of treatment resistance in pediatric depression. It depicts our proposed stages of treatment resistance in pediatric depression. These are extrapolated based on similar guidelines in adult depression but adjusted for the different levels of depression in pediatric depression (Ruhe, van Rooijen, Spijker, Peeters, & Schene, 2012; Sackeim et al., 2019). Based on clinical circumstances (e.g., acute suicidality and comorbidity), practitioners may want to skip or reorder certain treatment modalities. This table is meant for illustrative purposes and is not meant as an algorithm to guide treatment decisions for individual cases. Abbreviations: ECT, electroconvulsive therapy; FDA, Food and Drug Administration; rTMS, repetitive transcranial magnetic stimulation; SNRI, selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor
While multiple strategies for producing a consensus definition of treatment resistance in psychiatric disorders exist (Bokma et al., 2019), the definitions provided in Box 1 mirror the adult depression literature on this topic but are adapted for the reduced pediatric depression evidence base. Given the lack of evidence for the advanced pharmacologic and psychotherapeutic strategies listed for Stages 6 through 10, this proposed staging model is somewhat conservative in attempting to ensure that a patient has genuinely received evidence-based treatments before their symptoms are declared treatment-resistant or treatment-refractory. There is natural tension between exercising caution about prematurely marking cases as treatment-resistant given the real risks and adverse effects of medications largely untested in pediatric populations, versus wanting to quickly identify and aggressively treat treatment-resistant patients as soon as possible to prevent losing important developmental time on ineffective treatments. There is some evidence that introducing earlier adjunctive interventions is helpful in treatment-resistant patients, with improved outcomes in 10 nonremitting TORDIA subjects who received antipsychotic or mood stabilizer augmentation compared with 153 nonremitting subjects who did not (Emslie et al., 2010). However, more rigorous data that explicitly test this hypothesis in adequately powered trials are urgently needed.
Thus, there is no substitute for careful clinical formulation and judgment for each individual case, and there may be instances when patients may rightly progress more quickly through this proposed staging schema (Figure 2). For example, patients with severe depressive symptoms or persistent suicidality, who have multiple risk factors for treatment resistance, could reasonably be triaged for accelerated advancement to the less evidence-based augmentation and interventional strategies once past a full trial of an SSRI and evidence-based psychotherapy.
In determining where a child may fall in the stages of treatment resistance, it is important to differentiate between partial and failed responses. In general, a full response is defined as a 50% reduction in depression severity (typically as measured by the CDRS-R), with remission defined as a CDRS-R score less than 28 (Poznanski et al., 1984). Responses are considered partial if they fall between a 25% and 50% reduction in symptoms, and nonresponses are considered a less than 25% reduction in symptoms. In clinical practice, it is not uncommon to encounter patients who initially experience a significant improvement from a medication during the first weeks or months, but subsequently relapse. Families might report ‘gaining a tolerance’ to a medication that showed initial, but not sustained, efficacy. The question of whether brief initial responses represent expectancy rather than true pharmacologic effects is unclear, and it may be reasonable to consider these scenarios as partial responses. Regardless, in the event of a partial response, reasonable actions to take include increasing the dose as tolerated, adding psychotherapy if it has not already been added (and can increase the psychotherapy dose as well), and maximizing the family and psychosocial milieu (e.g., accommodations for school and treatment for depressed parents).
Strategies for treatment-resistant depression
Here, we discuss treatment strategies for pediatric TRD, defined as children or adolescents who are experiencing significant depressive symptoms that result in distress or impairment despite receiving an adequate trial of both psychotherapy and a first-line antidepressant agent (an SSRI such as fluoxetine, escitalopram, or sertraline; Box 1, Figure 2). TRD is relatively common (with 30%–40% of children) failing to achieve treatment response to first-line interventions defined by at least a 50% reduction in symptoms (Brent et al., 2008; March et al., 2004). Adolescents who do not respond to first-line treatments for depression have higher suicide rates, greater academic and social impairments, greater relationship problems with family and peers, and a greater likelihood of recurrence of depression in adulthood (Maalouf, Atwi, & Brent, 2011). Unfortunately, there is scant research examining the comparative efficacy of different available treatments for TRD in adolescents, despite how common and debilitating the condition is in pediatric populations. Thus, we draw considerably on the adult TRD literature and discuss potential applications to pediatric populations.
Given that (a) the side effects associated with second-line treatments are often more substantial, (b) the developmental cost of inadequately treated depression for many children is often considerable and linked to persisting impairment in adulthood (Dunn & Goodyer, 2006), and (c) the evidence regarding the benefits of these treatments are much less certain, it is absolutely imperative to (a) conduct a thorough evaluation to reassess alternative explanations for the depressive symptoms (trauma, untreated anxiety, bullying, etc.) and (b) to ensure the adequacy of delivery of first-line treating including evaluating adherence, as above.
Similar to TRD in adults, when first-line pharmacological and behavioral treatments have failed to produce adequate symptom relief for children and families, four primary treatment options remain available: (a) medication switch; (b) pharmacologic augmentation strategies, (c) psychotherapy augmentation strategies, and (4) interventional depression treatments. As in adult TRD guidelines, augmentation strategies are generally preferred when children exhibit a partial response (>25% improvement in depression symptoms but still inadequate symptom relief) to initial medication treatment and medication switching is a generally preferred strategy if a child experiences no response (<25% improvement in symptoms) or significant side effects on initial pharmacotherapy.
Medication switch
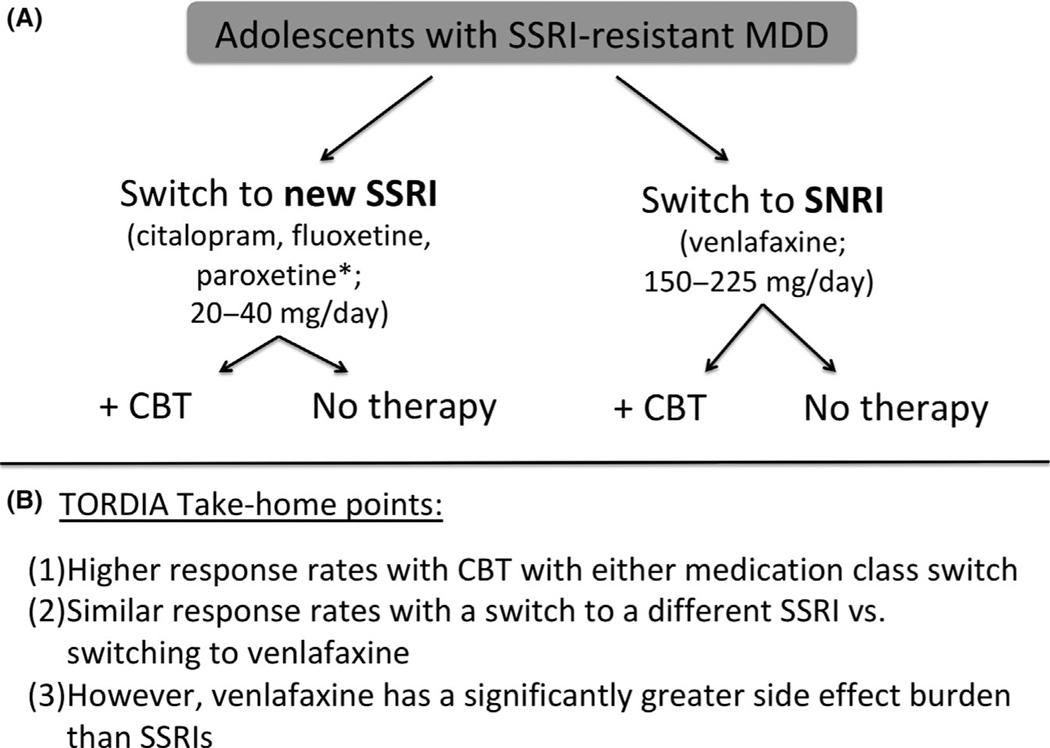
The guidance for failed responses comes almost exclusively from the TORDIA trial, as the only randomized, controlled treatment trial that has examined the comparative efficacy of different treatment strategies for SSRI-resistant depression in adolescents (Brent et al., 2008). TORDIA compared the efficacy of four different treatment strategies in 334 SSRI-resistant adolescents (Figure 3).
Figure 3.
TORDIA study methods and findings. (A) It depicts the principal methodology of the TORDIA trial with SSRI-resistant patients randomized to an SSRI switch with or without CBT or a switch to the SNRI, venlafaxine, with or without CBT. (B) Three major take-home points are listed here, with a more detailed description listed in the text. *Paroxetine was discontinued midway through the TORDIA trial in favor of citalopram. Abbreviations: CBT, cognitive-behavioral therapy; MDD, major depressive disorder; SNRI, selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TORDIA, Treatment of SSRI-Resistant Depression in Adolescents
TORDIA demonstrated that (a) receiving CBT while switching to either a new SSRI or the SNRI, venlafaxine, led to higher response rates (55% vs. 41%, p < .01); (b) switching to a different SSRI and switching to venlafaxine demonstrated similar response rates (47% vs 48%, p = .83), but (c) switching to venlafaxine was associated with a significantly greater increase in diastolic blood pressure and heart rate than switching to a different SSRI (Brent et al., 2008). The increase in blood pressure and heart rate associated with venlafaxine (and not SSRI) treatment, although statistically significant, rarely led to serious adverse events. Switching to a different SSRI rather than switching to venlafaxine was ultimately recommended given the similar efficacy between treatments and the increased hemodynamic side effects observed with venlafaxine (Brent et al., 2008). TORDIA emphasized the importance of delivering evidence-based CBT to SSRI-refractory pediatric MDD patients and provided the encouraging evidence that the response rate of even many ‘treatment-resistant’ adolescents provided with systematic evidence-based treatment is substantial – 55% of the children receiving CBT and 41% of the children receiving only evidence-based pharmacotherapy responded within 12 weeks. The high response rate even of adolescents who did not receive CBT in TORDIA (or in pediatric depression trials in general) emphasizes the potential importance of systematic and rigorous assessment of depression symptoms in helping to improve short-term treatment outcomes. Specifically, regular medication management visits with a physician as well as regular structured assessment of symptoms with rating scales may lead to improved outcomes, even in treatment-refractory patients (Palpacuer et al., 2017).
The only other published randomized, controlled pharmacological trial for pediatric TRD besides TORDIA was a small, underpowered placebo-controlled trial of 27 adolescent inpatients with treatment-refractory depression (defined by being symptomatic for 3 months and having been treated with an SSRI, tricyclic, or lithium), which failed to demonstrate the efficacy of the tricyclic antidepressant, amitriptyline, over the 10-week trial (Birmaher et al., 1998). Thus, beyond the TORDIA-based recommendation to approach failed trials with a switch to a different SSRI (preferred over venlafaxine given the more benign side effect profile; Brent et al., 2008), there is little additional evidence to guide decision making.
Many medications with evidence of efficacy in adult depression have failed to demonstrate significant benefits in pediatric populations. Non-SSRI antidepressants that have failed to show significant differences from placebo in clinical trials include venlafaxine (Emslie, Findling, Yeung, Kunz, & Li, 2007), mirtazapine (Ryan, 2005), duloxetine (Atkinson et al., 2014; Emslie et al., 2014), tricyclic antidepressants (Hazell & Mirzaie, 2013), and selegiline (DelBello et al., 2014). However, there have been substantial methodological limitations associated with these trials that may have weakened signal detection. These trial limitations are suggested by the observation that, when active comparators such as fluoxetine are included in these antidepressant trials, fluoxetine (a medication with substantial evidence of efficacy for pediatric depression) has generally failed to separate from both placebo and the investigated medications (Emslie et al., 2014). Other commonly used medications in adult depression that have not been studied in controlled trials in pediatric depression include bupropion, desvenlafaxine, vilazodone, trazodone, or vortioxetine.
Pharmacologic augmentation strategies
When a child exhibits a partial, but not complete response to initial SSRI pharmacotherapy, adding another medication is often the preferred pharmacological strategy. Commonly utilized augmentation treatments in adult depression include antipsychotics (primarily second generation), bupropion, buspirone, mirtazapine, lithium, and thyroid hormone. Before the evidence supporting these pharmacological treatment strategies are discussed in depth, it is important to note that the most evidence-based augmentation strategy in adolescent depression is CBT based on TORDIA (Brent et al., 2008).
There are no randomized, placebo-controlled studies examining the efficacy of antipsychotic augmentation for treatment-refractory pediatric depression. Although a secondary analysis of 6-month TORDIA outcomes suggested a potential benefit of earlier augmentation with an atypical antipsychotic or mood stabilizer, numbers were small (5 of 10 subjects improved with augmentation vs. 27 of 153 improved without; Emslie et al., 2010) and more pediatric evidence is sorely needed. Meta-analysis of adult studies suggests that antipsychotic augmentation is more effective than placebo for improving treatment-refractory depression (Zhou, Keitner, et al., 2015). However, the treatment benefit of antipsychotic augmentation in adult treatment-refractory depression is modest (effect size: 0.27–0.41) and there is no evidence of differential efficacy between antipsychotic medications (Zhou, Keitner, et al., 2015; Zhou, Ravindran, et al., 2015). Network meta-analysis of randomized controlled trials of treatment-refractory depression in adults suggests a similar modest benefit of atypical antipsychotics with odds ratio of treatment response ranging from 1.40 to 1.92 (Zhou, Ravindran, et al., 2015). Based on data in pediatric trials of psychosis and aggression, it is clear that antipsychotic medications have substantial side effects in children including weight gain, increased risk of metabolic syndrome, and diabetes as well as akathisia, tardive dyskinesia (Pagsberg et al., 2017; Stafford et al., 2015; Young, Taylor, & Lawrie, 2015). The overall prescribing rate of second-generation antipsychotics in pediatric populations has considerably increased, with more than a sixfold increase in the percentage of total office visits associated with antipsychotic prescription from the mid-1990s compared with the mid-2000s (Olfson, Blanco, Liu, Wang, & Correll, 2012). Antipsychotic prescription continued to rise between 2006 and 2010 in pediatric populations, with depression accounting for a substantial portion of visit diagnoses, particularly in young adults (Olfson, King, & Schoenbaum, 2015).
Similarly, there are no randomized, placebo-controlled trials examining the efficacy of lithium as a monotherapy or augmentation strategy in pediatric depression (Duffy & Grof, 2018). Meta-analysis of placebo-controlled trials of lithium augmentation in adult depression suggests that the odds ratio of a treatment response was 2.89 (95% CI: 1.65–5.05; Nelson, Baumann, Delucchi, Joffe, & Katona, 2014). The treatment benefit of lithium augmentation is much lower when active comparator trials are included in network meta-analysis with an estimated OR of 1.56 (95% CI: 1.05–2.55; Zhou, Ravindran, et al., 2015). Additionally, the emerging evidence that lithium may be an effective medication in reducing suicidality and self-harm in adult (Cipriani, Hawton, Stockton, & Geddes, 2013; Smith & Cipriani, 2017) and adolescent populations (Hafeman et al., 2019) make lithium a particularly enticing medication to further study in pediatric depression. The need for regular blood monitoring, the narrow therapeutic window, and the long-term risks of thyroid and kidney toxicity have limited the clinical use of lithium, especially in pediatric populations.
Omega-3 fatty acid supplementation has been well-studied in adult depression with meta-analyses suggesting a minimal-to-no benefit of omega-3 supplementation compared with placebo (Appleton, Sallis, Perry, Ness, & Churchill, 2015; Bloch & Hannestad, 2012; Nasir & Bloch, 2019). There is also strong evidence of publication bias suggesting that these small benefits may be inflated. An initial randomized, placebo-controlled trial in children with depression suggested a benefit of omega-3 fatty acids compared with placebo. This trial reported that over 16 weeks, omega-3 fatty acid supplementation demonstrated significant benefit compared with placebo in the 20 of 28 children who completed at least 1 month of treatment (Nemets, Nemets, Apter, Bracha, & Belmaker, 2006). More recently, in a larger trial of 51 medication-free adolescents with depression, omega-3 fatty acid supplementation did not demonstrate benefits for adolescent depression compared with placebo over 10 weeks of treatment (Gabbay et al., 2018). Open-label studies have examined omega-3 supplementation as a treatment strategy in SSRI-resistant adolescent depression (McNamara et al., 2014). Examination of erythrocyte omega-3 fatty acid levels suggested that 20 adolescents with SSRI-resistant depression had lower levels of docosahexaenoic acid (DHA) but not eicosapentaenoic acid (EPA). Supplementation with high doses of omega-3 fatty acids in these adolescents with SSRI-resistant depression led to significant reductions in depressive symptoms over 10 weeks of treatment (McNamara et al., 2014). The preliminary evidence suggesting possible benefits of omega-3 fatty acid supplementation should be viewed with significant skepticism given the quite substantial adult depression literature that suggests minimal-to-no benefit of omega-3 fatty acids in the treatment of depression (Nasir & Bloch, 2019).
Similarly, folic acid supplementation has been studied in multiple trials of adult depression with meta-analyses suggesting minimal-to-no benefit compared with placebo (Almeida, Ford, & Flicker, 2015; Roberts, Carter, & Young, 2018; Sarris et al., 2016; Schefft, Kilarski, Bschor, & Kohler, 2017). However, initial case report-level data in adolescents with treatment-refractory depression suggest that folate deficiency may play a role in refractory symptoms, with improvement by folate supplementation (Dartois, Stutzman, & Morrow, 2019). Specifically, case series have suggested that some adolescents have mutations in methylenetetrahydrofolate reductase (MTHFR) gene variants that reduce its activity (Dartois et al., 2019), and a subsequent study in adolescents with refractory depression examined cerebrospinal fluid (CSF) folate levels, showing that a proportion of these patients may have cerebral folate deficiency characterized by normal serum folate levels and low CSF 5-methyltetrahydrofolate (5-MTHF) levels (Pan et al., 2017). Both groups of treatment-refractory depressed adolescents showed significant improvement in depressive symptoms over time with folate supplementation (Dartois et al., 2019; Pan et al., 2017).
Table 2 summarizes the current evidence supporting the use of common augmentation strategies in adult treatment-refractory depression and their estimated treatment benefit compared with placebo in adult populations. We hope that synthesis of this evidence will be useful to practicing child psychiatrists, although any extrapolation to use in pediatric populations should be conducted with caution and with recognition of its limitations and the largely unstudied risks.
Table 2.
Augmentation strategies for treatment-resistant depression
| Response |
Time to effect |
Level of evidence for efficacy |
|||||
|---|---|---|---|---|---|---|---|
| Intervention | Odds ratio | NNT | Duration of trials | Time to maximum effect | Adults | Pediatrics | Adverse effects |
| Pharmacological augmentation strategies | |||||||
| Antipsychotics | 1.68 | 8 | 6–12 Weeks | 6–8 Weeks | A | D | Weight gain, metabolic syndrome, and diabetes |
| Lithium | 1.56 | 10 | 1–6 weeks | 4 Weeks | A | D | Nausea, vomiting, tremor, ataxia, kidney, and thyroid dysfunction |
| Thyroid hormone | 1.84 | 7 | 12 weeks | 6–8 weeks | B | D | Heat intolerance, anxiety, excessive sweating, insomnia, increased heart rate |
| Bupropion | 1.29 | 18 | 6–12 weeks | 4 Weeks | B | D | Headache, insomnia, anorexia, anxiety, tremor, risk of seizure |
| Buspirone | 1.25 | 20 | 4–12 weeks | 4 Weeks | B | D | Drowsiness, dizziness, nausea, headache |
| Lamotrigine | 1.12 | 41 | 8 weeks | 8 weeks | C | D | Drowsiness, nausea/vomiting, tremors, Steven–Johnson syndrome (rare), rash |
| Psychostimulants | 1.37 | 14 | 1–4 weeks | 2 weeks | A | D | Insomnia, anorexia, anxiety, psychosis, headache, increased blood pressure, abuse potential |
| Interventional treatments | |||||||
| ECT | 8.91 | 2 | 4 weeks | 4 Weeks | A | D | Headache, memory loss, confusion, medical complications of anesthesia |
| rTMS | 1.72 | 8 | 4–6 weeks | 4 Weeks | A | D | Headache, local scalp discomfort, and muscle twitching, lightheadedness, seizure (rare) |
| Ketamine | 8.97 | 2 | 2 Weeks | 1 Day | A | D | Dissociative effects, nausea/vomiting, increased blood pressure, potential for abuse |
It depicts pharmacological treatments for treatment-refractory depression in adults and their measured treatment benefit in network meta-analysis of adult randomized controlled trials (Zhou, Ravindran, et al., 2015) of adult treatment-refractory depression. Current levels of evidence of efficacy in both adult and pediatric populations are also reported based on grades developed from the National Guidelines Clearinghouse (AHRQ; Shekelle et al., 1999). Grade A evidence is based on meta-analysis of randomized controlled trial (RCT) data or 1 or more RCTs. Grade B evidence is based on at least 1 controlled trial that was not randomized. Grade C evidence is based on either data from nonexperimental studies or extrapolated from Grade A or Grade B evidence in a different population. Grade D is based on expert opinion or clinical experience. OR, odds ratio; NNT, number needed to treat. NNT is calculated based on the OR of Zhou, Ravindran et al. (2015), Nelson et al. (2014), and McIntyre et al. (2017), or other cited references and assuming a control response rate of 30% similar to response rate in Phase II augmentation arm of STAR*D. Grades of evidence based on Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing clinical guidelines. West J Med. 170(6):348–51, 1999 June.
Psychotherapy augmentation strategies
While CBT has been the best-studied specialized psychotherapy in adolescent populations, it is equally effective to IPT (Weersing et al., 2017), STPP, and a brief psychosocial intervention (Goodyer et al., 2017). There is no clear evidence to guide decisions regarding which type of psychotherapy might be most appropriate or engaging to an individual patient, and is an area that needs additional empiric study in both pediatric psychiatry and adult psychiatry. NICE guidelines suggest heavily involving the patient and his or her family in choosing a psychotherapy, which includes providing psychoeducation regarding the different school of thoughts, and their conceptions of dysfunction and recovery (National Institute for Health & Care Excellence, 2019). If a patient has failed an adequate trial of psychotherapy, it may be reasonable to switch to a different type of psychotherapy. It is also unclear whether increasing the dose of psychotherapy to a more intensive schedule (e.g., multiple sessions per week) is effective in the pediatric TRD population. These types of studies are just now being performed in a rigorous way in adult TRD populations, with promising results for more intensive or longer-term psychotherapies. Long-term psychoanalytic psychotherapy over 18 months was compared treatment as usual in a sample of adults with TRD, and while differences were not apparent at the end of the treatment phase, they emerged during the 24-, 30-, and 42-month follow-ups, with improvements in both the clinical and functional outcomes of patients who received psychoanalytic therapy (Fonagy et al., 2015). An additional study of chronically depressed adults in Germany compared long-term psychoanalytic therapy (36 months) with long-term CBT (15 months) and found significant reduction in depressive symptoms in both groups (Leuzinger-Bohleber et al., 2019).
Interventional treatments
Repetitive transcranial magnetic stimulation is currently approved as a treatment for treatment-resistant depression in adults. Meta-analysis of adult studies suggests that at the end of an acute treatment series the risk ratios for a treatment response are 1.72 (95% CI: 1.13–2.62) with a small effect size of 0.33 (95% CI 0.17–0.50) compared with sham treatment (Ontario, 2016). Uncontrolled studies of rTMS in adolescent depression have suggested possible efficacy and safety of rTMS (Bloch et al., 2008; Croarkin et al., 2018; MacMaster et al., 2019), but there are no controlled trials. Side effects of rTMS in pediatric patients appear similar to adults and include headache, scalp pain, and rare seizures (Magavi, Reti, & Vasa, 2017). Sham-controlled trials of rTMS for pediatric depression are currently ongoing (NCT01804270).
There are no published randomized controlled trials of electroconvulsive therapy in adolescents or children with MDD or TRD. Meta-analysis of adult ECT trials suggests strong evidence of efficacy for ECT compared to sham treatments with an odds ratio of treatment response of 8.91 (95% CI: 2.57–30.91) and effect size of 0.90 (95% CI: 0.52–1.27) compared to sham treatments (Kho, van Vreeswijk, Simpson, & Zwinderman, 2003; Mutz et al., 2019). Given the lack of data on efficacy and safety of ECT in pediatric populations, ECT is generally considered in adolescent depression after at least 3–4 failed antidepressant trials and at least one substantial psychotherapy trial (Birmaher et al., 2007; Brent & Birmaher, 2006).
There are currently no randomized controlled trials evaluating the efficacy of ketamine in children or adolescents with depression. Ketamine has been demonstrated to be an effective, rapidly acting treatment for adults with major depressive disorder (MDD) in multiple randomized, controlled trials. Meta-analysis of single-dose, controlled crossover trial of intravenous ketamine (0.5 mg/kg over 40 min) in adults with treatment-refractory depression suggests that more than half of adults with MDD who are given a single ketamine infusion experience a greater than 50% reduction in depressive symptoms within 1 day (Newport et al., 2015). Meta-analysis of controlled studies suggests an odds ratio of treatment response at 1 day following infusion of 9.87 (4.37–22.29) and an effect size of slightly over 1 [ES = 1.01 (95% CI: 0.69–1.34)] (Lee, Della Selva, Liu, & Himelhoch, 2015). Although the benefits of ketamine typically dissipate within a week or two, further research has suggested that when ketamine is given twice a week for several weeks, it can induce a prolonged treatment response (Singh et al., 2016). Meta-analysis of ketamine crossover trials also demonstrates significant antisuicidal properties (Wilkinson et al., 2018). Initial case reports (Dwyer et al., 2017) and a small open-label trial (Cullen et al., 2018) have suggested possible efficacy in pediatric populations, although there are currently no published randomized, controlled trials evaluating efficacy in pediatric populations. Recently, the FDA has approved esketamine (an intranasally delivered formulation of the l-enantiomer of ketamine) for the treatment of treatment-refractory depression in adults (Singh et al., 2016) and initial studies have suggested that esketamine may also be effective acutely in reducing suicidal ideation (Canuso et al., 2018). Trials examining the efficacy of ketamine (NCT02579928 and NCT03889756) and esketamine (NCT03185819) in pediatric depression populations are currently ongoing.
Conclusion
Given the significant differences both between adolescent and adult depression discussed earlier and their possible differences in response to available treatments, it remains a substantial and largely untested assumption that treatment-refractory depression in adolescents or children will respond similarly to pharmacological or somatic treatments as adults. Adolescent depression, even when properly treated, is a substantial cause of morbidity and mortality, including suicide. Suicide is the second leading cause of death in this age group (CDC, 2017), and MDD is the leading cause of disability worldwide (Lepine & Briley, 2011). In Figure 2, we have proposed a treatment-resistant staging system along with recommendations for increasing levels of severity, understanding that the evidence base thins considerably as patients move closer to more resistant and severe stages. In order to improve and advance the treatment of depression in adolescents, (a) randomized, placebo-controlled trials are needed to demonstrate that currently commonly utilized treatments for treatment-refractory depression (e.g., antipsychotic augmentation) is even effective; (b) controlled trials examining the efficacy of emerging and interventional treatments with large treatment effects in adults (e.g., ECT and ketamine) are urgently needed to help guide clinical practice. In the absence of these controlled trials, using electronic health records and adolescent cohort studies with propensity score matching might be useful approach to begin to identify the most efficacious interventions in clinical practice. Perhaps more importantly, clear and accepted definitions of treatment resistance and adequate trials of pharmacological and behavioral treatments are needed. These advances would help clinicians take a more systematic and evidence-based approach in treating this challenging condition. Additionally, greater access to and dissemination of evidence-based pharmacological and behavioral treatments for pediatric depression are needed as even basic, adequate initial behavioral and pharmacological treatments for depression are not available for most of the world’s children.
Acknowledgements
J.B.D. receives research support from the Klingenstein Third Generation Foundation. J.B.D. was supported on a T32 (MH018268) during the early phases of manuscript preparation. D.A.B. receives research support from NIMH, the VA, AFSP, the Once Upon a Time Foundation, and the Beckwith Foundation, and receives royalties from Guilford Press, from the electronic self-rated version of the C-SSRS from eRT, Inc., and from performing duties as an UptoDate Psychiatry Section Editor. D.A.B. also receives consulting fees from Healthwise. M.H.B. receives research support from Therapix Biosciences, Neurocrine Biosciences, Janssen Pharmaceuticals, and Biohaven Pharmaceuticals. M.H.B. gratefully acknowledges additional research support from NIH, NARSAD, Fund for Lesbian and Gay Studies at Yale, and the Patterson Foundation. The remaining authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: See Acknowledgements for full disclosures.
References
- Almeida OP, Ford AH, & Flicker L. (2015). Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. International Psychogeriatrics, 27, 727–737. [DOI] [PubMed] [Google Scholar]
- APA (2013). Diagnostic and statistical manual of mental disorders (5th edn). Arlington, VA: Author. [Google Scholar]
- Appleton KM, Sallis HM, Perry R, Ness AR, & Churchill R. (2015). Omega-3 fatty acids for depression in adults. Cochrane Database of Systematic Reviews, 11, Cd004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson SD, Prakash A, Zhang Q, Pangallo BA, Bangs ME, Emslie GJ, & March JS (2014). A double-blind efficacy and safety study of duloxetine flexible dosing in children and adolescents with major depressive disorder. Journal of Child and Adolescent Psychopharmacology, 24, 180–189. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Perel JM, Birmaher B, Rudolph GR, Nuss S, Bridge J, & Brent DA (2002). Sertraline pharmacokinetics and dynamics in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 1037–1044. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Faedda GL, Offidani E, Vazquez GH, Marangoni C, Serra G, & Tondo L. (2013). Antidepressant-associated mood-switching and transition from unipolar major depression to bipolar disorder: A review. Journal of Affective Disorders, 148, 129–135. [DOI] [PubMed] [Google Scholar]
- Beijers L, Wardenaar KJ, van Loo HM, & Schoevers RA (2019). Data-driven biological subtypes of depression: Systematic review of biological approaches to depression sub-typing. Molecular Psychiatry, 24, 888–900. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent D, Bernet W, Bukstein O, Walter H, Benson RS, ... & Medicus J. (2007). Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 1503–1526. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Waterman GS, Ryan ND, Perel J, McNabb J, Balach L, ... & Rao U. (1998). Randomized, controlled trial of amitriptyline versus placebo for adolescents with “treatment-resistant” major depression. Journal of the American Academy of Child and Adolescent Psychiatry, 37, 527–535. [DOI] [PubMed] [Google Scholar]
- Bloch MH, & Hannestad J. (2012). Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Molecular Psychiatry, 17, 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Y, Grisaru N, Harel EV, Beitler G, Faivel N, Ratzoni G, ... & Levkovitz Y. (2008). Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: An open-label study. The Journal of ECT, 24, 156–159. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, & Wisner KL (2005). Nutrition and depression: Implications for improving mental health among childbearing-aged women. Biological Psychiatry, 58, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokma WA, Wetzer G, Gehrels JB, Penninx B, Batelaan NM, & van Balkom A. (2019). Aligning the many definitions of treatment resistance in anxiety disorders: A systematic review. Depression and Anxiety, 36, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, & Birmaher B. (2006). Treatment-resistant depression in adolescents: Recognition and management. Child and Adolescent Psychiatric Clinics of North America, 15, 1015–1034. [DOI] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, ... & Zelazny J. (2008). Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA, 299, 901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, ... & Keller M. (2010). Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. American Journal of Psychiatry, 167, 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Perper JA, Goldstein CE, Kolko DJ, Allan MJ, Allman CJ, & Zelenak JP (1988). Risk factors for adolescent suicide. A comparison of adolescent suicide victims with suicidal inpatients. Archives of General Psychiatry, 45, 581–588. [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, & Brent DA (2009). Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. American Journal of Psychiatry, 166, 42–49. [DOI] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, ... & Brent DA (2007). Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA, 297, 1683–1696. [DOI] [PubMed] [Google Scholar]
- Canuso CM,Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, ... & Drevets WC (2018). Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: Results of a double-blind, randomized, placebo-controlled study. American Journal of Psychiatry, 175, 620–630. [DOI] [PubMed] [Google Scholar]
- Carton L, Pignon B, Baguet A, Benradia I, Roelandt JL, Vaiva G, ... & Rolland B. (2018). Influence of comorbid alcohol use disorders on the clinical patterns of major depressive disorder: A general population-based study. Drug and Alcohol Dependence, 187, 40–47. [DOI] [PubMed] [Google Scholar]
- CDC (2017). United States Mortality Tables by 5y age, race, sex (1999–2014). National Vital Statistics System. Available from: https://www.cdc.gov/nchs/nvss/mortality/lcwk1.htm [Google Scholar]
- Chanen AM, Jovev M, & Jackson HJ (2007). Adaptive functioning and psychiatric symptoms in adolescents with borderline personality disorder. Journal of Clinical Psychiatry, 68, 297–306. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Hawton K, Stockton S, & Geddes JR (2013). Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. BMJ, 346, f3646. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, ... & Xie P. (2016). Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet, 388, 881–890. [DOI] [PubMed] [Google Scholar]
- Clayborne ZM, Varin M, & Colman I. (2019). Systematic review and meta-analysis: Adolescent depression and long-term psychosocial outcomes. Journal of the American Academy of Child and Adolescent Psychiatry, 58, 72–79. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP, Perel JM, & Staron V. (2007). A pilot randomized controlled trial of combined trauma-focused CBT and sertraline for childhood PTSD symptoms. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 811–819. [DOI] [PubMed] [Google Scholar]
- Cook RJ, & Sackett DL (1995). The number needed to treat: A clinically useful measure of treatment effect. BMJ, 310, 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, & Blader JC (2015). Antipsychotic use in youth without psychosis: A double-edged sword. JAMA Psychiatry, 72, 859–860. [DOI] [PubMed] [Google Scholar]
- Coryell W, Scheftner W, Keller M, Endicott J, Maser J, & Klerman GL (1993). The enduring psychosocial consequences of mania and depression. American Journal of Psychiatry, 150, 720–727. [DOI] [PubMed] [Google Scholar]
- Craske MG, Farchione TJ, Allen LB, Barrios V, Stoyanova M, & Rose R. (2007). Cognitive behavioral therapy for panic disorder and comorbidity: More of the same or less of more? Behavior Research and Therapy, 45, 1095–1109. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Deng ZD, Romanowicz M, Voort JLV, Camsari DD, ... & Lewis CP (2018). High-frequency repetitive TMS for suicidal ideation in adolescents with depression. Journal of Affective Disorders, 239, 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Amatya P, Roback MG, Albott CS, Westlund Schreiner M, Ren Y, ... & Klimes-Dougan B. (2018). Intravenous ketamine for adolescents with treatment-resistant depression: An open-label study. Journal of Child and Adolescent Psychopharmacology, 28, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry J, Rohde P, Simons A, Silva S, Vitiello B, Kratochvil C, ... & March J. (2006). Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). Journal of the American Academy of Child and Adolescent Psychiatry, 45, 1427–1439. [DOI] [PubMed] [Google Scholar]
- D’Amico EJ, Parast L, Shadel WG, Meredith LS, Seelam R, & Stein BD (2018). Brief motivational interviewing intervention to reduce alcohol and marijuana use for at-risk adolescents in primary care. Journal of Consulting and Clinical Psychology, 86, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartois LL, Stutzman DL, & Morrow M. (2019). L-methylfolate augmentation to antidepressants for adolescents with treatment-resistant depression: A case series. Journal of Child and Adolescent Psychopharmacology, 29, 386–391. [DOI] [PubMed] [Google Scholar]
- Davey CG, Chanen AM, Hetrick SE, Cotton SM, Ratheesh A, Amminger GP, ... & Berk M. (2019). The addition of fluoxetine to cognitive behavioural therapy for youth depression (YoDA-C): A randomised, double-blind, placebo-controlled, multicentre clinical trial. Lancet Psychiatry, 6, 735–744. [DOI] [PubMed] [Google Scholar]
- Daviss WB, Birmaher B, Melhem NA, Axelson DA, Michaels SM, & Brent DA (2006). Criterion validity of the Mood and Feelings Questionnaire for depressive episodes in clinic and non-clinic subjects. Journal of Child Psychology and Psychiatry, 47, 927–934. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Augenstein TM, Wang M, Thomas SA, Drabick DAG, Burgers DE, & Rabinowitz J. (2015). The validity of the multi-informant approach to assessing child and adolescent mental health. Psychological Bulletin, 141, 858–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello MP, Hochadel TJ, Portland KB, Azzaro AJ, Katic A, Khan A, & Emslie G. (2014). A double-blind, placebo-controlled study of selegiline transdermal system in depressed adolescents. Journal of Child and Adolescent Psychopharmacology, 24, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson ET, Bloch MH, & Strawn JR (2019). Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: A network meta-analysis. Journal of Clinical Psychiatry, 80, 12064. [DOI] [PubMed] [Google Scholar]
- Duffy A, & Grof P. (2018). Lithium treatment in children and adolescents. Pharmacopsychiatry, 51, 189–193. [DOI] [PubMed] [Google Scholar]
- Dunn V, & Goodyer IM (2006). Longitudinal investigation into childhood- and adolescence-onset depression: Psychiatric outcome in early adulthood. British Journal of Psychiatry, 188, 216–222. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Beyer C, Wilkinson ST, Ostroff RB, Qayyum Z, & Bloch MH (2017). Ketamine as a treatment for adolescent depression: A case report. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 352–354. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, & Bloch MH (2019). Antidepressants for pediatric patients. Current Psychiatry, 18, 26–42. [PMC free article] [PubMed] [Google Scholar]
- Eckshtain D, Kuppens S, Ugueto A, Ng MY, Vaughn-Coaxum R, Corteselli K, & Weisz JR (2019). Meta-analysis: 13-Year follow-up of psychotherapy effects on youth depression. Journal of the American Academy of Child and Adolescent Psychiatry, 59, 45–63. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Findling RL, Yeung PP, Kunz NR, & Li Y. (2007). Venlafaxine ER for the treatment of pediatric subjects with depression: Results of two placebo-controlled trials. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 479–488. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Kennard BD, Mayes TL, Nakonezny PA, Zhu L, Tao R, ... & Croarkin P. (2012). Insomnia moderates outcome of serotonin-selective reuptake inhibitor treatment in depressed youth. Journal of Child and Adolescent Psychopharmacology, 22, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD, ... & Brent D. (2010). Treatment of Resistant Depression in Adolescents (TORDIA): Week 24 outcomes. American Journal of Psychiatry, 167, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Prakash A, Zhang Q, Pangallo BA, Bangs ME, & March JS (2014). A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder. Journal of Child and Adolescent Psychopharmacology, 24, 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekadu A, Wooderson S, Donaldson C, Markopoulou K, Masterson B, Poon L, & Cleare AJ (2009). A multidimensional tool to quantify treatment resistance in depression: The Maudsley staging method. Journal of Clinical Psychiatry, 70, 177–184. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, & Woodward LJ (2002). Mental health, educational, and social role outcomes of adolescents with depression. Archives of General Psychiatry, 59, 225–231. [DOI] [PubMed] [Google Scholar]
- Fonagy P, Rost F, Carlyle JA, McPherson S, Thomas R, Pasco Fearon RM, ... & Taylor D. (2015). Pragmatic randomized controlled trial of long-term psychoanalytic psychotherapy for treatment-resistant depression: The Tavistock Adult Depression Study (TADS). World Psychiatry, 14, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Freed RD, Alonso CM, Senger S, Stadterman J, Davison BA, & Klein RG (2018). A Double-Blind Placebo-Controlled Trial of Omega-3 fatty acids as a monotherapy for adolescent depression. Journal of Clinical Psychiatry, 79, 11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Asher G, Gartlehner G, Hoffman V, Green J, Boland E, ... & Lohr KN (2018). AHRQ technology assessments definition of treatment-resistant depression in the Medicare population. Rockville, MD: Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- Gilmore KJ, & Meersand P. (2013). Normal child and adolescent development: A psychodynamic primer (1st edn, pp. 203–282). Washington: American Psychiatric Publishing. [Google Scholar]
- Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, ... & Harrington R. (2008). A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial. Health Technology Assessment, 12, iii–iv, ix–60. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Reynolds S, Barrett B, Byford S, Dubicka B, Hill J, ... & Fonagy P. (2017). Cognitive behavioural therapy and short-term psychoanalytical psychotherapy versus a brief psychosocial intervention in adolescents with unipolar major depressive disorder (IMPACT): A multicentre, pragmatic, observer-blind, randomised controlled superiority trial. Lancet Psychiatry, 4, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, & Wilkinson PO (2019). Practitioner Review: Therapeutics of unipolar major depressions in adolescents. Journal of Child Psychology and Psychiatry, 60, 232–243. [DOI] [PubMed] [Google Scholar]
- Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, ... & Mathers CD (2011). Global burden of disease in young people aged 10–24 years: A systematic analysis. Lancet, 377, 2093–2102. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, & Kessler RC (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). Journal of Clinical Psychiatry, 76, 155–162. [DOI] [PubMed] [Google Scholar]
- Guile JM, Boissel L, Alaux-Cantin S, & de La Riviere SG (2018). Borderline personality disorder in adolescents: Prevalence, diagnosis, and treatment strategies. Adolescent Health, Medicine and Therapeutics, 9, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson JG, & Links P. (2014). Handbook of good psychiatric management for borderline personality disorder (1st edn). Washington: American Psychiatric Publishing. [Google Scholar]
- Guo T, Xiang YT, Xiao L, Hu CQ, Chiu HF, Ungvari GS, ... & Wang G. (2015). Measurement-based care versus standard care for major depression: A Randomized Controlled Trial with blind raters. American Journal of Psychiatry, 172, 1004–1013. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Rooks B, Merranko J, Liao F, Gill MK, Goldstein TR, ... & Birmaher B. (2019). Lithium versus other mood stabilizing medications in a longitudinal study of bipolar youth. Journal of the American Academy of Child and Adolescent Psychiatry, 10.1016/j.jaac.2019.06.013 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad TA, Laughren T, & Racoosin J. (2006). Suicidality in pediatric patients treated with antidepressant drugs. Archives of General Psychiatry, 63, 332–339. [DOI] [PubMed] [Google Scholar]
- Hazell P, & Mirzaie M. (2013). Tricyclic drugs for depression in children and adolescents. Cochrane Database of Systematic Reviews, 6, Cd002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiligenstein JH, Hoog SL, Wagner KD, Findling RL, Galil N, Kaplan S, ... & Jacobson JG (2006). Fluoxetine 40–60 mg versus fluoxetine 20 mg in the treatment of children and adolescents with a less-than-complete response to nine-week treatment with fluoxetine 10–20 mg: A pilot study. Journal of Child and Adolescent Psychopharmacology, 16, 207–217. [DOI] [PubMed] [Google Scholar]
- Jain S, Carmody TJ, Trivedi MH, Hughes C, Bernstein IH, Morris DW, ... & Rush AJ (2007). A psychometric evaluation of the CDRS and MADRS in assessing depressive symptoms in children. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, & Bloch MH (2016). Systematic Review and Meta-Analysis: Dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. American Journal of Psychiatry, 173, 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe RT (1997). Refractory depression: Treatment strategies, with particular reference to the thyroid axis. Journal of Psychiatry and Neuroscience, 22, 327–331. [PMC free article] [PubMed] [Google Scholar]
- Kaess M, von Ceumern-Lindenstjerna IA, Parzer P, Chanen A, Mundt C, Resch F, & Brunner R. (2013). Axis I and II comorbidity and psychosocial functioning in female adolescents with borderline personality disorder. Psychopathology, 46, 55–62. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Martin A, King RA, & Charney D. (2001). Are child-, adolescent-, and adult-onset depression one and the same disorder? Biological Psychiatry, 49, 980–1001. [DOI] [PubMed] [Google Scholar]
- Keenan-Miller D, Hammen CL, & Brennan PA (2007). Health outcomes related to early adolescent depression. Journal of Adolescent Health, 41, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Ellis SP, Gorlyn M, Burke AK, Oquendo MA, Mann JJ, & Grunebaum MF (2018). Suicidal ideation declines with improvement in the subjective symptoms of major depression. Journal of Affective Disorders, 227, 65–70. [DOI] [PubMed] [Google Scholar]
- Kennard BD, Clarke GN, Weersing VR, Asarnow JR, Shamseddeen W, Porta G, ... & Brent DA (2009). Effective components of TORDIA cognitive-behavioral therapy for adolescent depression: Preliminary findings. Journal of Consulting and Clinical Psychology, 77, 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard BD, Silva SG, Tonev S, Rohde P, Hughes JL, Vitiello B, ... & March J. (2009). Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): Acute and long-term outcomes. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, & Ries Merikangas K. (2001). Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry, 49, 1002–1014. [DOI] [PubMed] [Google Scholar]
- Kho KH, van Vreeswijk MF, Simpson S, & Zwinderman AH (2003). A meta-analysis of electroconvulsive therapy efficacy in depression. The Journal of ECT, 19, 139–147. [DOI] [PubMed] [Google Scholar]
- Kovacs M. (1985). The Children’s Depression, Inventory (CDI). Psychopharmacology Bulletin, 21, 995–998. [PubMed] [Google Scholar]
- Kronenberg S, Apter A, Brent D, Schirman S, Melhem N, Pick N, ... & Weizman A. (2007). Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. Journal of Child and Adolescent Psychopharmacology, 17, 741–750. [DOI] [PubMed] [Google Scholar]
- Lam RW, Parikh SV, Ramasubbu R, Michalak EE, Tam EM, Axler A, ... & Manjunath CV (2013). Effects of combined pharmacotherapy and psychotherapy for improving work functioning in major depressive disorder. British Journal of Psychiatry, 203, 358–365. [DOI] [PubMed] [Google Scholar]
- Lee EE, Della Selva MP, Liu A, & Himelhoch S. (2015). Ketamine as a novel treatment for major depressive disorder and bipolar depression: A systematic review and quantitative meta-analysis. General Hospital Psychiatry, 37, 178–184. [DOI] [PubMed] [Google Scholar]
- Lepine JP, & Briley M. (2011). The increasing burden of depression. Neuropsychiatric Disease and Treatment, 7 (Suppl. 1), 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger-Bohleber M, Hautzinger M, Fiedler G, Keller W, Bahrke U, Kallenbach L, ... & Beutel M. (2019). Outcome of psychoanalytic and cognitive-behavioural long-term therapy with chronically depressed patients: A controlled trial with preferential and randomized allocation. Canadian Journal of Psychiatry, 64, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, & Rohde P. (1994). Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child and Adolescent Psychiatry, 33, 809–818. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, & Andrews JA (1993). Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. Journal of Abnormal Psychology, 102, 133–144. [DOI] [PubMed] [Google Scholar]
- Lewis CC, Simons AD, & Kim HK (2012). The role of early symptom trajectories and pretreatment variables in predicting treatment response to cognitive behavioral therapy. Journal of Consulting and Clinical Psychology, 80, 525–534. [DOI] [PubMed] [Google Scholar]
- Maalouf FT, Atwi M, & Brent DA (2011). Treatment-resistant depression in adolescents: Review and updates on clinical management. Depress Anxiety, 28, 946–954. [DOI] [PubMed] [Google Scholar]
- Maalouf FT, Porta G, Vitiello B, Emslie G, Mayes T, Clarke G, ... & Brent D. (2012). Do sub-syndromal manic symptoms influence outcome in treatment resistant depression in adolescents? A latent class analysis from the TORDIA study. Journal of Affective Disorders, 138, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Croarkin PE, Wilkes TC, McLellan Q, Langevin LM, Jaworska N, ... & Kirton A. (2019). Repetitive transcranial magnetic stimulation in youth with treatment resistant major depression. Frontiers in Psychiatry, 10, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magavi LR, Reti IM, & Vasa RA (2017). A review of repetitive transcranial magnetic stimulation for adolescents with treatment-resistant depression. International Review of Psychiatry, 29, 79–88. [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, . .. & Severe J. (2004). Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA, 292, 807–820. [DOI] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, & Emslie GJ (2010). Psychometric properties of the Children’s Depression Rating Scale-Revised in adolescents. Journal of Child and Adolescent Psychopharmacology, 20, 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Lee Y, Zhou AJ, Rosenblat JD, Peters EM, Lam RW,... Jerrell JM (2017). The Efficacy of Psychostimulants in Major Depressive Episodes: A Systematic Review and Meta-Analysis. Journal of Clinical Psychopharmacology, 37, 412–418. [DOI] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, ... & Brent DA (2012). Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Strimpfel J, Jandacek R, Rider T, Tso P, Welge JA, ... & Delbello MP (2014). Detection and treatment of long-chain omega-3 fatty acid deficiency in adolescents with SSRI-resistant major depressive disorder. PharmaNutrition, 2, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Muehlenkamp JJ, & Jacobson CM (2008). Fact or fiction: Diagnosing borderline personality disorder in adolescents. Clinical Psychology Review, 28, 969–981. [DOI] [PubMed] [Google Scholar]
- Moretti MM, Fine S, Haley G, & Marriage K. (1985). Childhood and adolescent depression: Child-report versus parent-report information. Journal of the American Academy of Child Psychiatry, 24, 298–302. [DOI] [PubMed] [Google Scholar]
- Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CHY, & Young AH (2019). Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: Systematic review and network meta-analysis. BMJ, 364, 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R, & Danese A. (2012). Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. American Journal of Psychiatry, 169, 141–151. [DOI] [PubMed] [Google Scholar]
- Nardi B, Francesconi G, Catena-Dell’osso M, & Bellan-tuono C. (2013). Adolescent depression: Clinical features and therapeutic strategies. European Review for Medical and Pharmacological Sciences, 17, 1546–1551. [PubMed] [Google Scholar]
- Nasir M, & Bloch MH (2019). Trim the fat: The role of omega-3 fatty acids in psychopharmacology. Therapeutic Advances in Psychopharmacology, 9, 2045125319869791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2019). Depression in children and young people: Identification and management. NICE guidelines. Available from: https://www.nice.org.uk/guidance/ng134/chapter/Recommendations-step-3-managing-mild-depression [PubMed] [Google Scholar]
- Nelson JC, Baumann P, Delucchi K, Joffe R, & Katona C. (2014). A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. Journal of Affective Disorders, 168, 269–275. [DOI] [PubMed] [Google Scholar]
- Nemets H, Nemets B, Apter A, Bracha Z, & Belmaker RH (2006). Omega-3 treatment of childhood depression: A controlled, double-blind pilot study. American Journal of Psychiatry, 163, 1098–1100. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, & Nemeroff CB (2015). Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. American Journal of Psychiatry, 172, 950–966. [DOI] [PubMed] [Google Scholar]
- Nichter B, Norman S, Haller M, & Pietrzak RH (2019). Psychological burden of PTSD, depression, and their comorbidity in the U.S. veteran population: Suicidality, functioning, and service utilization. Journal of Affective Disorders, 256, 633–640. [DOI] [PubMed] [Google Scholar]
- Offidani E, Fava GA, Tomba E, & Baldessarini RJ (2013). Excessive mood elevation and behavioral activation with antidepressant treatment of juvenile depressive and anxiety disorders: A systematic review. Psychotherapy and Psychosomatics, 82, 132–141. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu SM, Wang S, & Correll CU (2012). National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Archives of General Psychiatry, 69, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Olfson M, King M, & Schoenbaum M. (2015). Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry, 72, 867–874. [DOI] [PubMed] [Google Scholar]
- Ontario HQ (2016). Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis of Randomized Controlled Trials. Ontario Health Technology Assessment Series, 16, 1–66. [PMC free article] [PubMed] [Google Scholar]
- Pagsberg AK, Tarp S, Glintborg D, Stenstrom AD, Fink-Jensen A, Correll CU, & Christensen R. (2017). Acute antipsychotic treatment of children and adolescents with schizophrenia-spectrum disorders: A systematic review and network meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 191–202. [DOI] [PubMed] [Google Scholar]
- Palpacuer C, Gallet L, Drapier D, Reymann JM, Falissard B, & Naudet F. (2017). Specific and non-specific effects of psychotherapeutic interventions for depression: Results from a meta-analysis of 84 studies. Journal of Psychiatric Research, 87, 95–104. [DOI] [PubMed] [Google Scholar]
- Pan LA, Martin P, Zimmer T, Segreti AM, Kassiff S, McKain BW, ... & Vockley J. (2017). Neurometabolic disorders: Potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. American Journal of Psychiatry, 174, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, & Gibbons R. (1984). Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child Psychiatry, 23, 191–197. [DOI] [PubMed] [Google Scholar]
- Puccio F, Fuller-Tyszkiewicz M, Ong D, & Krug I. (2016). A systematic review and meta-analysis on the longitudinal relationship between eating pathology and depression. International Journal of Eating Disorders, 49, 439–454. [DOI] [PubMed] [Google Scholar]
- Rengasamy M, Mansoor BM, Hilton R, Porta G, He J, Emslie GJ, ... & Brent DA (2013). The bi-directional relationship between parent-child conflict and treatment outcome in treatment-resistant adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry, 52, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes MM, Panza KE, Martin A, & Bloch MH (2011). Time-lag bias in trials of pediatric antidepressants: A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Riglin L, Lomax T, Souter E, Potter R, Smith DJ, ... & Thapar A. (2019). Adolescent and adult differences in major depression symptom profiles. Journal of Affective Disorders, 243, 175–181. [DOI] [PubMed] [Google Scholar]
- Rice F, Sellers R, Hammerton G, Eyre O, Bevan-Jones R, Thapar AK, ... & Thapar A. (2017). Antecedents of new-onset major depressive disorder in children and adolescents at high familial risk. JAMA Psychiatry, 74, 153–160. [DOI] [PubMed] [Google Scholar]
- Roberts E, Carter B, & Young AH (2018). Caveat emptor: Folate in unipolar depressive illness, a systematic review and meta-analysis. Journal of Psychopharmacology, 32, 377–384. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, van Rooijen G, Spijker J, Peeters FP, & Schene AH (2012). Staging methods for treatment resistant depression. A systematic review. Journal of Affective Disorders, 137, 35–45. [DOI] [PubMed] [Google Scholar]
- Russell ST, & Fish JN (2016). Mental health in lesbian, gay, bisexual, and transgender (LGBT) youth. Annual Review of Clinical Psychology, 12, 465–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ND (2005). Treatment of depression in children and adolescents. Lancet, 366, 933–940. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Aaronson ST, Bunker MT, Conway CR, Demitrack MA, George MS, ... & Rushi AJ (2019). The assessment of resistance to antidepressant treatment: Rationale for the Antidepressant Treatment History Form: Short Form (ATHF-SF). Journal of Psychiatric Research, 113, 125–136. [DOI] [PubMed] [Google Scholar]
- Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, & Ng CH (2016). Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. American Journal of Psychiatry, 173, 575–587. [DOI] [PubMed] [Google Scholar]
- Schefft C, Kilarski LL, Bschor T, & Kohler S. (2017). Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. European Neuropsychopharmacology, 27, 1090–1109. [DOI] [PubMed] [Google Scholar]
- Scott K, Lewis CC, & Marti CN (2019). Trajectories of symptom change in the treatment for adolescents with depression study. Journal of the American Academy of Child and Adolescent Psychiatry, 58, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra G, Uchida M, Battaglia C, Casini MP, De Chiara L, Biederman J, ... & Wozniak J. (2017). Pediatric Mania: The controversy between euphoria and irritability. Current Neuropharmacology, 15, 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddeen W, Asarnow JR, Clarke G, Vitiello B, Wagner KD, Birmaher B, ... & Brent DA (2011). Impact of physical and sexual abuse on treatment response in the Treatment of Resistant Depression in Adolescent Study (TORDIA). Journal of the American Academy of Child and Adolescent Psychiatry, 50, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddeen W, Clarke G, Keller MB, Wagner KD, Birmaher B, Emslie GJ, ... & Brent DA (2012). Adjunctive sleep medications and depression outcome in the treatment of serotonin-selective reuptake inhibitor resistant depression in adolescents study. Journal of Child and Adolescent Psychopharmacology, 22, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Ha C, Michonski J, Venta A, & Carbone C. (2012). Borderline personality disorder in adolescents: Evidence in support of the Childhood Interview for DSM-IV Borderline Personality Disorder in a sample of adolescent inpatients. Comprehensive Psychiatry, 53, 765–774. [DOI] [PubMed] [Google Scholar]
- Sharp C, & Wall K. (2018). Personality pathology grows up: Adolescence as a sensitive period. Current Opinion in Psychology, 21, 111–116. [DOI] [PubMed] [Google Scholar]
- Shekelle PG, Woolf SH, Eccles M, & Grimshaw J. (1999). Clinical guidelines: Developing guidelines. BMJ, 318, 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, ... & Van Nueten L. (2016). A Double-Blind, Randomized, Placebo-Controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. American Journal of Psychiatry, 173, 816–826. [DOI] [PubMed] [Google Scholar]
- Smith KA, & Cipriani A. (2017). Lithium and suicide in mood disorders: Updated meta-review of the scientific literature. Bipolar Disorders, 19, 575–586. [DOI] [PubMed] [Google Scholar]
- Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O, ... & Mendlewicz J. (1999). Treatment resistant depression: Methodological overview and operational criteria. European Neuropsychopharmacology, 9, 83–91. [DOI] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Stafford MR, Mayo-Wilson E, Loucas CE, James A, Hollis C, Birchwood M, & Kendall T. (2015). Efficacy and safety of pharmacological and psychological interventions for the treatment of psychosis and schizophrenia in children, adolescents and young adults: A systematic review and meta-analysis. PLoS ONE, 10, e0117166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, & Rynn MA (2015). Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depression and Anxiety, 32, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A. (2017). Editorial: What is depression? Journal of Child Psychology and Psychiatry, 58, 1287–1289. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Baroni A, Haimm C, Brotman M, Lowe CH, Myers F, ... & Leibenluft E. (2010). Pediatric bipolar disorder versus severe mood dysregulation: Risk for manic episodes on follow-up. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 397–405. [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Cohen P, Pine DS, & Leibenluft E. (2009). Adult outcomes of youth irritability: A 20-year prospective community-based study. American Journal of Psychiatry, 166, 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas P, Brotman MA, & Leibenluft E. (2018). Practitioner Review: Definition, recognition, and treatment challenges of irritability in young people. Journal of Child Psychology and Psychiatry, 59, 721–739. [DOI] [PubMed] [Google Scholar]
- Swartz HA, Cyranowski JM, Cheng Y, Zuckoff A, Brent DA, Markowitz JC, ... & Frank E. (2016). Brief psychotherapy for maternal depression: Impact on mothers and children. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 495–503.e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, & Thase ME (2009). Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: A meta-analysis including 6562 patients. Journal of Clinical Psychiatry, 70, 344–353. [DOI] [PubMed] [Google Scholar]
- Torres Soler C, Olofsdotter S, Vadlin S, Ramklint M, Nilsson KW, & Sonnby K. (2018). Diagnostic accuracy of the Montgomery-Asberg Depression Rating Scale parent report among adolescent psychiatric outpatients. Nordic Journal of Psychiatry, 72, 184–190. [DOI] [PubMed] [Google Scholar]
- Towbin K, Vidal-Ribas P, Brotman MA, Pickles A, Miller KV, Kaiser A, ... & Stringaris A. (2019). A Double-Blind Randomized Placebo-Controlled Trial of citalopram adjunctive to stimulant medication in youth with chronic severe irritability. Journal of the American Academy of Child and Adolescent Psychiatry, 10.1016/j.jaac.2019.05.015 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Morris DW, Wisniewski SR, Lesser I, Nierenberg AA, Daly E, ... & Rush AJ (2013). Increase in work productivity of depressed individuals with improvement in depressive symptom severity. American Journal of Psychiatry, 170, 633–641. [DOI] [PubMed] [Google Scholar]
- Trowell J, Joffe I, Campbell J, Clemente C, Almqvist F, Soininen M, ... & Tsiantis J. (2007). Childhood depression: A place for psychotherapy. An outcome study comparing individual psychodynamic psychotherapy and family therapy. European Child and Adolescent Psychiatry, 16, 157–167. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Dixon-Gordon KL, Austin SB, Rodriguez MA, Zachary Rosenthal M, & Chapman AL (2015). Non-suicidal self-injury with and without borderline personality disorder: Differences in self-injury and diagnostic comorbidity. Psychiatry Research, 230, 28–35. [DOI] [PubMed] [Google Scholar]
- van Geel M, Vedder P, & Tanilon J. (2014). Relationship between peer victimization, cyberbullying, and suicide in children and adolescents: A meta-analysis. JAMA Pediatrics, 168, 435–442. [DOI] [PubMed] [Google Scholar]
- Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, & Bloch MH (2015). Systematic Review and Meta-Analysis: Early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 557–564. [DOI] [PubMed] [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, & Stringaris A. (2016). The status of irritability in psychiatry: A conceptual and quantitative review. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT (2017). Antidepressant efficacy for depression in children and adolescents: Industry- and NIMH-funded studies. American Journal of Psychiatry, 174, 430–437. [DOI] [PubMed] [Google Scholar]
- Wallace AE, Neily J, Weeks WB, & Friedman MJ (2006). A cumulative meta-analysis of selective serotonin reuptake inhibitors in pediatric depression: Did unpublished studies influence the efficacy/safety debate? Journal of Child and Adolescent Psychopharmacology, 16, 37–58. [DOI] [PubMed] [Google Scholar]
- Weersing VR, Jeffreys M, Do MT, Schwartz KT, & Bolano C. (2017). Evidence base update of psychosocial treatments for child and adolescent depression. Journal of Clinical Child & Adolescent Psychology, 46, 11–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, Wisniewski SR, Fava M, ... & Rush AJ (2006). Remissions in maternal depression and child psychopathology: A STAR*D-child report. JAMA, 295, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, ... & Wickramaratne P. (1999). Depressed adolescents grown up. JAMA, 281, 1707–1713. [DOI] [PubMed] [Google Scholar]
- Weisz JR, Kuppens S, Ng MY, Eckshtain D, Ugueto AM, Vaughn-Coaxum R, ... & Fordwood SR (2017). What five decades of research tells us about the effects of youth psychological therapy: A multilevel meta-analysis and implications for science and practice. American Psychologist, 72, 79–117. [DOI] [PubMed] [Google Scholar]
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, & Boddington E. (2004). Selective serotonin reuptake inhibitors in childhood depression: Systematic review of published versus unpublished data. Lancet, 363, 1341–1345. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, ... & Sanacora G. (2018). The effect of a single dose of intravenous ketamine on suicidal ideation: A systematic review and individual participant data meta-analysis. American Journal of Psychiatry, 175, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, O’Connor EA, Eder M, & Whitlock EP (2009). Screening for child and adolescent depression in primary care settings: A systematic evidence review for the US Preventive Services Task Force. Pediatrics, 123, e716–e735. [DOI] [PubMed] [Google Scholar]
- Woldu H, Porta G, Goldstein T, Sakolsky D, Perel J, Emslie G, ... & Brent D. (2011). Pharmacokinetically and clinician-determined adherence to an antidepressant regimen and clinical outcome in the TORDIA trial. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2019). Disease Burdern and mortality estimates, 2000–2016. Available from: https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html
- Young SL, Taylor M, & Lawrie SM (2015). “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. Journal of Psychopharmacology, 29, 353–362. [DOI] [PubMed] [Google Scholar]
- Zhou X, Keitner GI, Qin B, Ravindran AV, Bauer M, Del Giovane C, ... & Xie P. (2015). Atypical antipsychotic augmentation for treatment-resistant depression: A systematic review and network meta-analysis. International Journal of Neuropsychopharmacology, 18, pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, ... & Xie P. (2015). Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: Systematic review and network meta-analysis. Journal of Clinical Psychiatry, 76, e487–e498. [DOI] [PubMed] [Google Scholar]