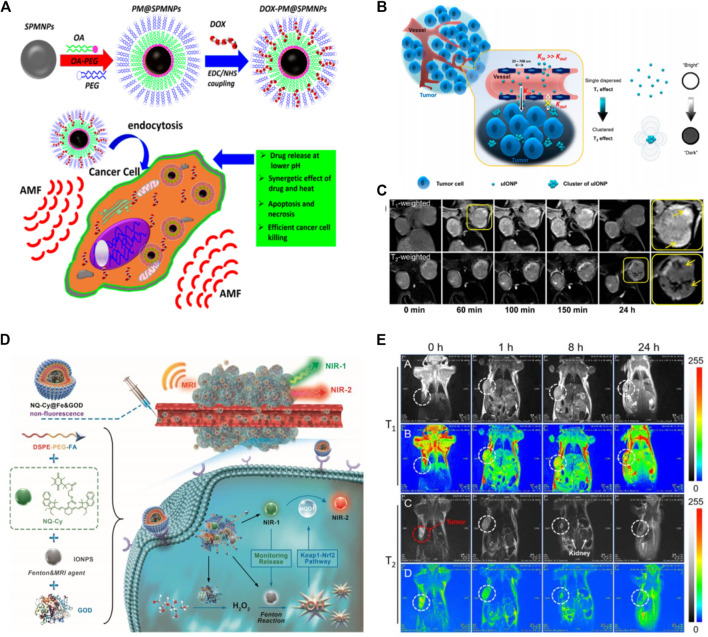
FIGURE 2.
Schematics (A) illustration of the anticancer drug (DOX) conjugating into polymer encapsulated superparamagnetic NPs and the overall mechanism of cancer cell killing by synergistic therapy under applied Alternating Magnetic Field (AMF). Copyright 2017, American chemical Society (Thorat et al., 2017) (B) Representation of the mechanism of improved EPR effect and accumulation of ultrafine iron oxide nanoparticles (uIONPs) inside tumor with a bright-to-dark T1-T2 MRI contrast transition. uIONPs quickly and easily extravasate from dripping tumor vessels into a tumor with favorable kinetics, then self-assemble into clusters in the tumor microenvironment at a low pH (6.5), limiting clustered uIONP intravasation back into circulation (C) Optical images of T1-and T2-weighted MRI of a mouse with orthotopic 4T1 tumors pre- and post i.v administration of uIONP at different time intervals (Wang L. et al., 2017). Copyright 2017, American Chemical Society (D) Tumor-specific nanotheranostics NQ-Cy@Fe&GOD for in situ real-time reporting of Fenton-based dose-dependent •OH generation: 1) Owing to the passive and active targeting ability, NQ-Cy@Fe&GOD are delivered into tumor sites, along with the enhanced MRI signal; 2) Turn-on NIR fluorescence signal at 830 nm (NIR-1) is observed in synchronous with its intracellular dissociation for evaluating the released dose of Fenton agents. Concurrently, the released GOD catalyzes glucose to produce H2O2; 3) Subsequently, high-level •OH is generated by IONPs-based Fenton reaction with intratumor H2O2, thereby triggering the activation of Keap1-Nrf2 pathway for high-level overexpression of NQO1 enzyme, which is timely supervised by a new emission at 650 nm (NIR-2). Overall, this nanocomposite bridges dual-channel NIR imaging and MRI modality, thereby uniting spatial and temporal resolution on dose-dependent •OH therapeutic feedback (E) Imaging of the bio-distribution with MRI signal by intravenous injection. T1-Weighted (A,B) and T2-Weighted (C,D) MRI of tumor-bearing mice after experiencing a magnetic field at after the intravenous administration of NQ-Cy@Fe&GOD at different time intervals. Copyright 2020, John Wiley and Sons (Ma et al., 2020).