Fig. 1.
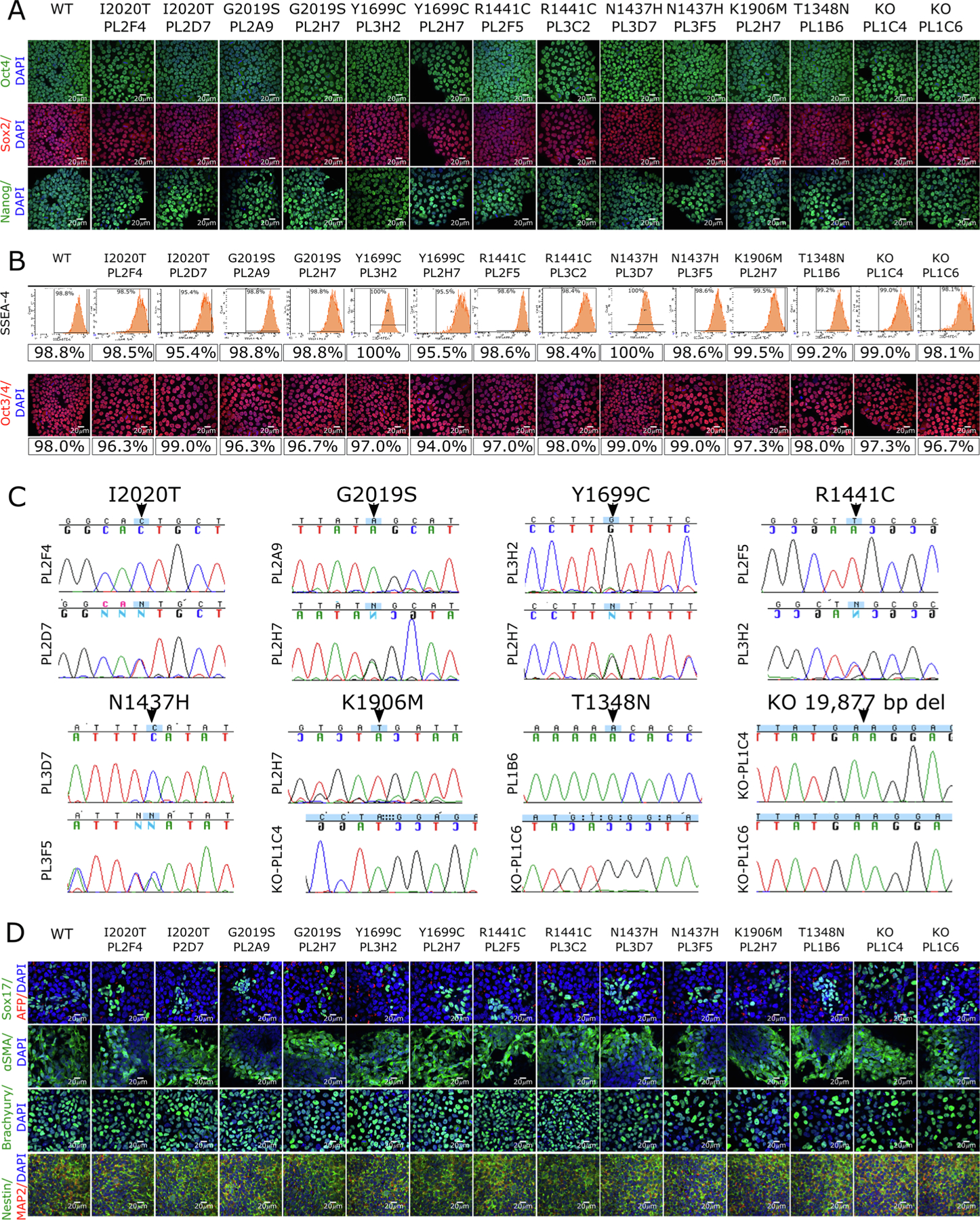
Generation of lines with LRRK2 mutations on an isogenic background. (A) iPSC lines carrying the indicated mutations (WT = wild type, KO = knockout; clone ID below each mutation) were stained for pluripotency markers OCT4 (top panels, green), SOX2 (middle panels, red) and NANOG (lower panels, green) and counterstained with the nuclear stain DAPI (blue, all panels). Scale bars = 20 μm. (B) Quantitative analysis of SSEA-4 expression by flow cytometry (upper panels) or by staining for Oct3/4 (lower panels; red is Oct3/4, blue is DAPI; Scale bars = 20 μm) allowed percentages of pluripotent cells to be estimated as indicated. (C) Sequence chromatograms of LRRK2 coding changes, where upper panels show homozygous and lower panels show heterozygous clones. (D) Differentiation potential was assessed using immunostaining for endodermal markers SOX17 (green, first row) and α-fetoprotein (AFP, red, first row); mesodermal markers alpha-muscle actin (SMA, green, second row) and Brachyury (green, third row); and ectodermal markers Nestin (green, fourth row) and MAP2 (red, fourth row). All cells were counterstained with the nuclear dye DAPI (blue) and scale bars = 20 μm.
