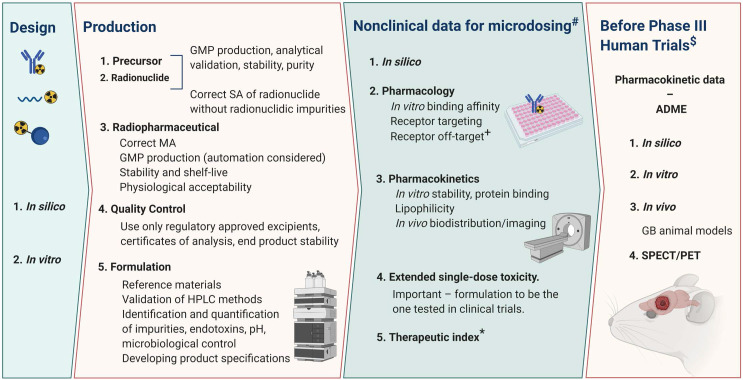
Figure 9.
Quality data required for translation of a radiopharmaceutical. The sequential approach to an adequate validation of radiopharmaceuticals is illustrated; certain tests and validation steps may not depend on each other and are therefore often performed in parallel. Abbreviated and footnoted content: Absorption Distribution Metabolism Excretion (ADME), good manufacturing practice (GMP), molar activity (MA), specific activity (SA), glioblastoma (GB), positron emission tomography (PET), single-photon emission computed tomography (SPECT). ($) i.e.: target validation, (*) a requirement only for the validation of therapeutic radiopharmaceuticals, (#) not required for microdosing e.g. radiopharmaceuticals (<100 µg); e.g. genotoxicity, safety pharmacology, repeat dose toxicity. (+) radiolabelling may alter the pharmacological characterisation of the targeting molecule; pharmacological effects should be ruled out at the anticipated clinical dose 247.