Table 1.
| Entry | Alkyne | R | 1,4-Regioisomers (CuAAC) (%) | 1,5-Regioisomers (RuAAC)b | Ratio 1,4/1,5 | |
|---|---|---|---|---|---|---|
| Δ | Δ | MW yield % (Conv. %) | ||||
| 1 | 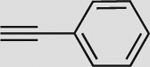 |
a | 86 | 79 | 88 (93) | 4:96 |
| 2 | 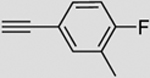 |
b | 88 | 83 | 92 (100) | 3:93 |
| 3 | 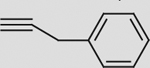 |
c | 93 | 71 | 92 (100) | 4:96 |
| 4 | 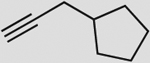 |
d | 87 | 79 | 87 (100) | 5:95 |
| 5 | e | 89 | 82 | 91 (96) | 4:96 | |
| 6 | f | 83 | 68 | 87 (100) | 5:95 | |
| 7 |  |
g | 92 | 77 | 95 (100) | 5:95 |
| 8 | h | 63 | 54 | 93 (100) | 3:97 | |
Conditions: azide 1 (1 equiv), alkyne (1.1 equiv), Cu powder (4 equiv), CuSO4·5H2O (0.2 equiv), tBuOH/H2O.
Conditions: azide 1 (1 equiv, 0.1 M in THF), alkyne (1.5 equiv), Cp*RuCl(PPh3)2 (0.05 equiv), THF, thermal heating (Δ): 6 h, T=50 °C or microwave conditions (MW): 5 min, T=100 °C.
