FIG A1.
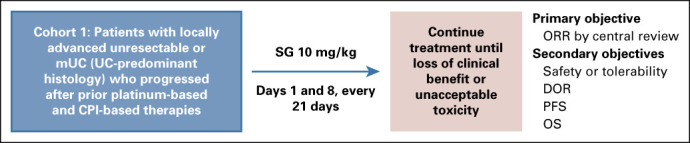
TROPHY-U-01 study design. EudraCT Number: 2018-001167-23; ClinicalTrials.gov identifier: NCT03547973; IMMU-132-06 study. CPI, checkpoint inhibitor; DOR, duration of response; mUC, metastatic urothelial cancer; ORR, objective response rate; OS, overall survival; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; SG, sacituzumab govitecan.
