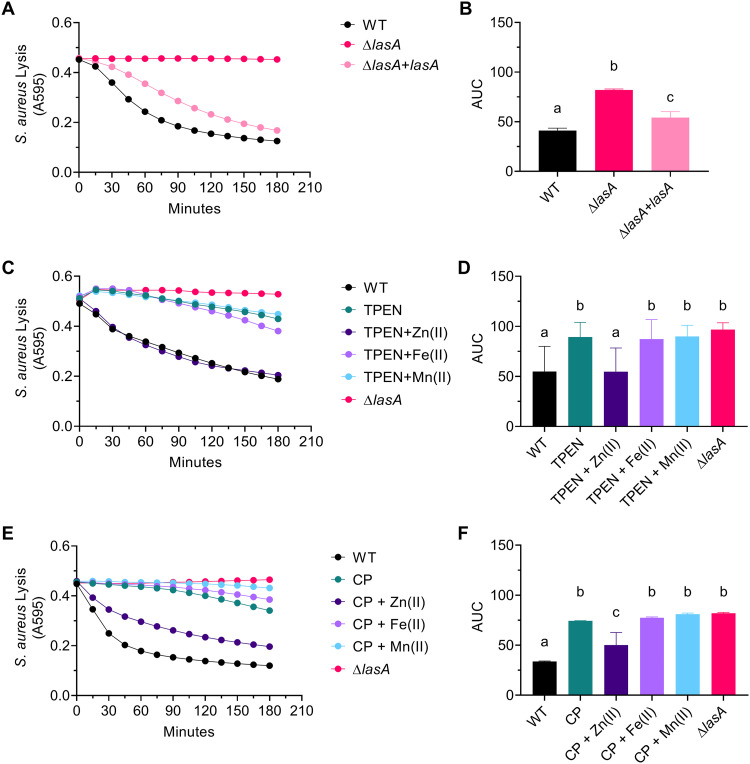
FIG 5.
Zinc chelation inhibits LasA enzymatic activity. (A and B) Lysis of heat-killed S. aureus strain SH1000 by cell-free supernatants from WT, ΔlasA, and ΔlasA+lasA (ΔlasA complemented in trans under the control of arabinose-inducible PBAD) 16 h cultures. (C and D) Lysis of heat-killed S. aureus strain SH1000 by WT and ΔlasA cell-free supernatants. WT supernatant was divided and left untreated (WT), treated with 50 μM TPEN (TPEN), or treated with 50 μM TPEN and 500 μM ZnSO4 · 7 H2O [TPEN+Zn(II)], (NH4)2Fe(SO4)2 · 6 H2O [TPEN+Fe(II)], or MnCl2 · 4 H2O [TPEN+Mn(II)]. (E and F) Lysis of heat-killed S. aureus strain SH1000 by WT and ΔlasA cell-free supernatants. WT supernatant was divided and left untreated (WT), treated with 40 μM CP (CP), or treated with 40 μM CP and 160 μM ZnSO4 · 7 H2O [CP+Zn(II)], (NH4)2Fe(SO4)2 · 6 H2O [CP+Fe(II)], or MnCl2 · 4 H2O [CP+Mn(II)]. (A, C, and E) The data represent the mean from three independent experiments. Error bars have been omitted for clarity. (B, D, and F) Quantification of data in panels A, C, and E, respectively, using area under the curve (AUC). Data are the mean ± SD from three independent experiments. Samples marked with the same letter are not significantly different; samples marked with different letters are significantly different (P < 0.05).