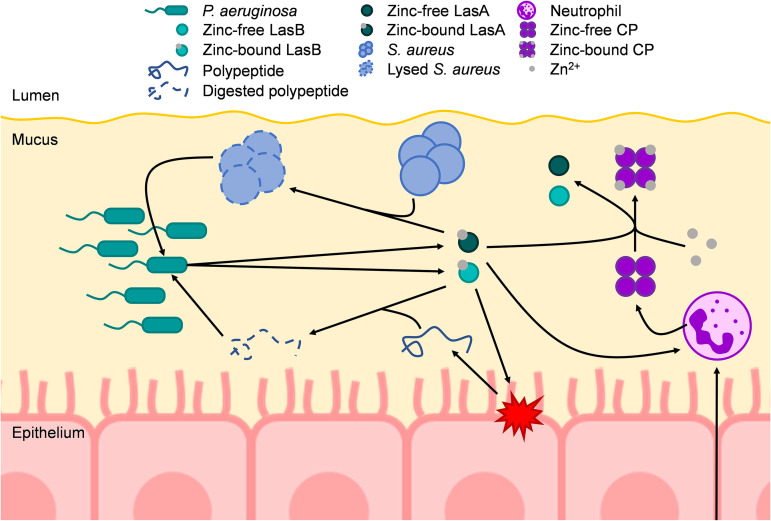
FIG 6.
Model of the effects of CP-mediated zinc chelation in the CF lung on P. aeruginosa. P. aeruginosa colonizes the mucus in the airways of CF patients to high densities, which in part requires the uptake and utilization of zinc. At high densities, P. aeruginosa secretes a variety of quorum sensing-dependent virulence factors, including zinc metalloproteases such as LasB and LasA. LasB is a protease that can degrade host proteins, such as elastin, as well as peptides. These degraded proteins/peptides can then be taken up and utilized as nutrients by P. aeruginosa. LasA is a protease that lyses S. aureus by cleaving pentaglycine bridges of peptidoglycan. LasA-mediated lysis of S. aureus allows P. aeruginosa to take up nutrients released from lysed S. aureus as well as to outcompete S. aureus in the CF lung. During infection, neutrophils are recruited to sites of infection/inflammation. Neutrophils may then release cellular contents such as CP. CP can then bind bioavailable zinc away from P. aeruginosa, thus reducing the overall abundance of P. aeruginosa, while also inducing a zinc starvation response by P. aeruginosa. Additionally, CP can bind zinc away from both LasB and LasA, thereby inhibiting their proteolytic activity. Furthermore, LasB and LasA activity have been shown to induce neutrophil extracellular traps (NETs). Therefore, CP-mediated inhibition of LasB and LasA activity may lead to less NET formation and, subsequently, less CP release. Arrows indicate a positive interaction.