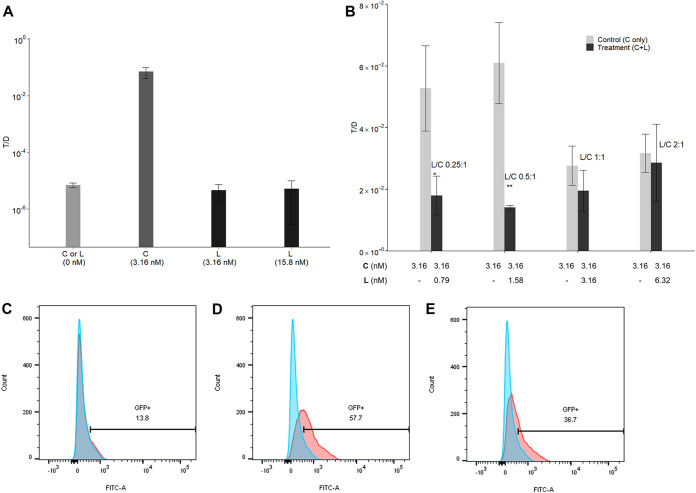
FIG 7.
Influence of L peptide on the conjugative transfer of pCF10. Donor cells were incubated with L peptide (L) (cCF10 fragment, sequence LVTL) alone or together with cCF10 (C) at 37°C for 30 min. Then, recipient cells were added and incubated for 10 min to allow one round of conjugation before the entire culture was plated on selective medium for transconjugants and donors. The conjugation frequency is represented by the number of transconjugants per donor (T/D ratio). (A) L was added at physiological (3.16 nM) or higher concentrations (15.8 nM) to induce donor cells. The groups induced with no peptide (L or C) or 3.16 nM C served as the negative-control and positive-control groups, respectively. The data are presented as the means ± SDs (n = 3). (B) Different volumes of 1 mg/ml L stock solution (dissolved in dimethyl sulfoxide [DMSO]) and a fixed volume of 1 mg/ml C stock solution (dissolved in DMSO; final conc., 3.16 nM) were added to achieve L/C ratios of 0.25:1, 0.5:1, 1:1, and 2:1 for induction of donor cells (treatment groups). The groups induced with 3.16 nM C served as the negative control. In addition, to eliminate the effect of DMSO, a volume of DMSO equal to that of the L stock solution was added to the negative control group. The data are presented as the means ± SDs (n = 3). *, P < 0.05; **, P < 0.01 compared with each negative control group (Student’s t test). (C to E) The expression of the cCF10-inducible GFP reporter harbored on pCF10 was quantified using flow cytometry. Blue peak, wild-type E. faecalis OG1RF that would not express the GFP reporter was used as the negative control. Red peaks, donor cells induced with no peptides for 0 min (C), 0.7 μl DMSO plus 3.16 nM C for 60 min (D), or 1.58 nM L plus 3.16 nM C (L/C = 1:2) for 60 min (E). The numbers shown in the middles of panels C to E are the percentages of GFP-positive cells after gating.