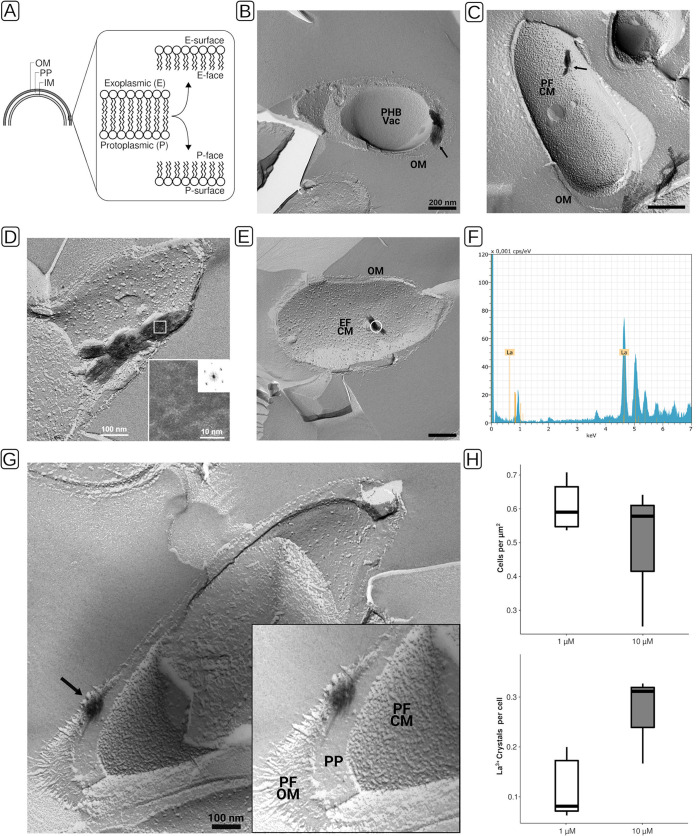
FIG 4.
Freeze fracture transmission electron microscopy (FFTEM), EDX analysis, and quantitation of lanthanum deposits of Beijerinckiaceae bacterium RH AL1 cultures grown with lanthanum. Biomass was rapidly frozen in a liquid ethane-propane mixture before freeze fracturing was done at −150°C in a BAF400T freeze fracture unit (BAL-TEC, Liechtenstein). Fractured samples were shadowed with 2 nm Pt/C (platinum/carbon), followed by perpendicular evaporation of a 15- to 20-nm-thick carbon layer. (A) Schematic for distinction of fracture faces in FFTEM micrographs. (B to E, G) Freeze fracture replicas were cleaned with commercial sodium hypochlorite solution before being transferred onto uncoated EM grids for examination by TEM. Black arrows indicate crystals that were found in proximity to the cytoplasmic and outer membranes (B to E) and in the periplasmic space (G). (B, D, E, G) TEM micrographs originating from cultures grown with 1 μM lanthanum. (C) TEM micrograph originating from a culture grown with 10 μM lanthanum. (D) The frequency spectra of crystals were calculated by fast Fourier transformation from digital images of frozen and fractured cells. (E) The sample area for EDX analysis is marked with a white circle. (F) The analysis was carried out as outlined for Fig. 1. (H) Lanthanum deposits were counted to determine the number of crystals per cell. For 1 μM lanthanum cultures, a total area of 233 μm2 (n = 5 areas of 46.6 μm2) was analyzed, and for 10 μM lanthanum cultures, a total area of 285.3 μm2 (n = 3 areas of 95.1 μm2) was analyzed. OM, outer membrane; PP, periplasm; IM, inner membrane; CM, cytoplasmic membrane; PF, P face; EF, E face; PHB, polyhydroxybutyrate; Vac, vacuole.