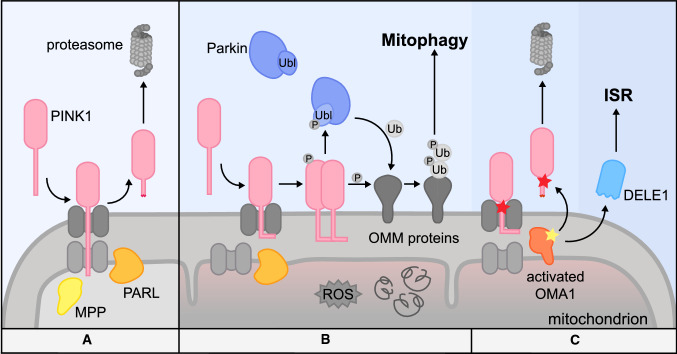
Fig. 3.
Regulation of the mitophagy factor PINK1 by different proteases. A Under homeostatic conditions, the N-terminus of PINK1 is imported through the TOM and TIM complex into the matrix where its MTS is cleaved by MPP. Additionally, PARL cleaves PINK1 at alanine 103 and the C-terminal fragment containing the kinase domain is retro-translocated to the cytosol for proteasomal degradation. B Severe mitochondrial stress stalls import across the IMM and disrupts PINK1 processing. This results in the stabilization of PINK1 on the OMM, presumably by lateral release from the TOM complex. Subsequently, PINK1 undergoes dimerization and activating autophosphorylation. Activated PINK1 phosphorylates ubiquitin entities on OMM proteins as well as a ubiquitin-like domain in Parkin. This results in a positive feedback reaction of PINK1/Parkin-dependent phospho-ubiquitination on the mitochondrial surface. OMM proteins modified in this manner recruit mitophagy receptors that coordinate the destruction of the organelle in lysosomes. C Certain PD-related PINK1 mutants fail to be stabilized on the OMM in response to mitochondrial stress. Instead, they are processed by OMA1, the mitoprotease which is also involved in the cleavage of DELE1 in ISR induction